Keywords
|
| Pulmonary Alveolar Proteinosis; Bronchoalveolar Carcinoma; Nonspecific or acute Interstitial Pneumonia |
Introduction
|
| Pulmonary Alveolar Proteinosis (PAP) is a rare but often reported diffuse pulmonary disease that is characterized by the collection of lipoproteinaceous material in the alveoli of the lungs which leads to impaired gas transfer [1]. This disease was first described as a distinct clinical disorder in 19581 by Rosen et al. More than 260 case reports have been reported since 1958 and small series have described more than 514 cases in the literature [2,3]. |
| Ramirez and Harlan in 1968 demonstrated that the accumulation of phospholipids in the alveolar space in PAP was due to its decreased clearance and not due to its increased synthesis. They also found that the nature of the phospholipid accumulated is chemically similar to that of the naturally occurring surfactant [4]. |
| This accumulation of phospholipids may be idiopathic [onset in adulthood], secondary [due to a specific exposure or any disorder] or congenital [in the neonatal period due to autosomal recessive inherited de?ciency] [2]. |
| More than 90% PAP cases are idiopathic PAP [5] which can indeed be classified as an autoimmune disorder [6]. In the 1990s, Knockout mice lacking either the gene for GM-CSF itself or for the β chain of the receptor were found to develop a pulmonary disorder similar to PAP [7,8]. |
| There is no specific medication that can be used to treat this condition and whole lung lavage is the only established standard therapy that can decrease the symptoms and improve the oxygenation in the affected patients [9] Whole lung lavage is however required only for symptomatic patients of PAP [especially in idiopathic PAP] and is particularly contraindicated in Congenital PAP [2]. The aim of treatment is symptomatic amelioration along with radiological and physiological improvement [10,11]. |
| Apart from WLL, the treatment for idiopathic PAP has evolved from using a variety of nonspecific unsuccessful agents [like antibiotics, corticosteroids, aerosolized trypsin, lavage with heparin and saline, mucolytic agent ambroxol or postural drainage] to the development of specific therapy targeted at the underlying pathogenesis of the disorder [2] This specific therapy is immune-modulatory, by either supplying exogenous GM-CSF [subcutaneous or inhalational] [12,13], or by inhibiting the CD20+ antibody forming cells, with Rituximab [14]. |
| Unlike WLL, GM-CSF is not effective in secondary PAP where the main treatment is removal of exposure or treating the underlying disorder [15]. |
| There is indeed no established order in the treatment of idiopathic PAP with WLL and GM-CSF therapy, though most centers have opted for GM- CSF therapy after sequential WLL. There are some studies that suggest GM-CSF therapy as an alternative to Whole lung lavage [16], the essence of the treatment of idiopathic PAP from most studies is the combination of both [17,18]. |
| We are presenting a case of Idiopathic PAP whom we have treated successfully with WLL followed by 8 weeks of GM-CSF therapy. |
Case Report
|
| We encountered a 53 years old normotensive, euglycemic gentleman who presented with a history of progressively increasing breathlessness for one and a half years. He also complained of a low grade fever, malaise and dry cough for three weeks. He was a driver in a transport company in Doha [Middle East] working for the past 7 years, was a non-smoker and had no other addiction. He hails from North India and is of average build and height. |
| On examination, he was dyspneic and his SpO2 was 78% at room air and 84% while on 6 l/min O2. ABG revealed pO2 of 52 (Table 1). There were coarse crepitations all over his chest bilaterally and no clubbing, cyanosis, pallor or lymphadenopathy. The differential diagnosis considered included Pneumocytis Carinii Pneumonia (PCP), Diffuse Alveolar Haemorrhage, Brachioalveolar Carcinoma (BAC), Protein Alveolar Proteinosis, Nonspecific or acute interstitial Pneumonia (NSIP/AIP), and pulmonary edema. Each of these diagnoses was systematically ruled out. He had no history of hemoptysis, weight loss or bronchorrhea or chest pain and HIV test was found to be non-reactive. ECG and Echocardiography were also unremarkable. |
| There is a history of a similar episode of breathlessness 2 years ago. At that time a bronchoscopic biopsy was done in Doha which showed Pulmonary Alveolar Proteinosis. Single lung lavage of the left lung was done at that time following which he was asymptomatic for 6 months. |
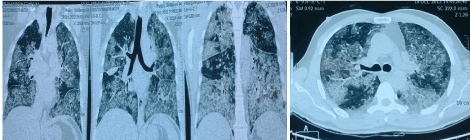
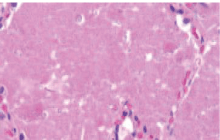
| On imaging studies, notably HRCT chest, there were bilateral patchy areas of ground glass opacities with thickening of the interlobular septae (Figure 1). This is commonly known as ‘crazy paving pattern’. On Bronchoscopy, PAS positive material was detected in the bronchial lavage (Figure 2). |
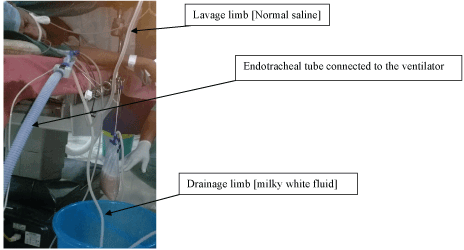
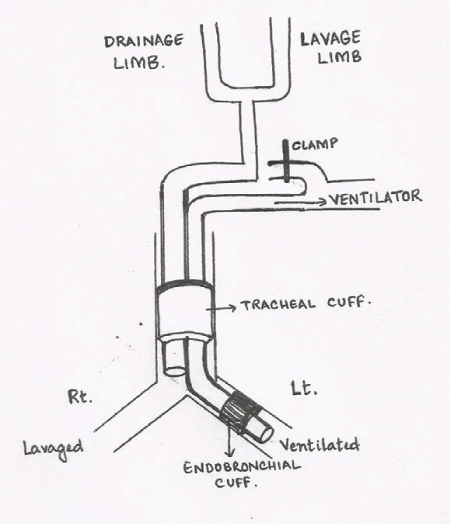
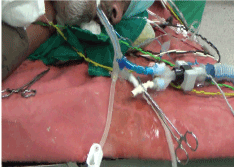
| Management for this patient was initially decided to be bilateral whole lung lavage. Since his right lung was affected more, single lung lavage of the right side was done first. At the operation theatre the patient was turned to right lateral decubitus position and the procedure was done under general anaesthesia. The double lumen endotracheal tube was introduced in such a way that the non-dependent lung [left] was ventilated by the ventilator via the endobronchial tube which was cuffed and separated from the opposite [right] lung. The dependent [right] lung was lavaged with the tracheal lumen which was connected to the lavage-drainage circuit. The tracheal cuff was inflated to separate the lungs from the environment (Figure 3 and 4). Flexible bronchoscopy was performed to confirm the appropriate tube placement. |
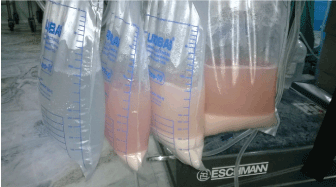
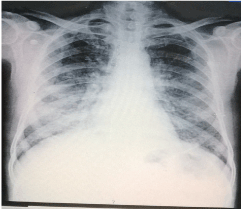
| Thereafter with the bed in reverse Trendelenburg position, 1 liter warm [around 35-40 degree Celsius] saline solution was introduced into the right lung. Chest physiotherapy was done by vibrating the chest with good grasp to the mid and lower thoracic region. Gradually as the bed was being brought to Trendelenburg position chest wall compression technique was used to bring maximum effluent from the lavaged lung. This was one cycle of lavage-drainage. During the procedure 36 liters saline water was used until clear effluent was seen (Figure 5). The procedure took more than 3 hours. Strict monitoring of each cycle of lavage and drainage was done so as to minimize residual fluid in the lavaged lung. The patient dramatically improved after the procedure and the next morning he was found to be quite relieved of his breathlessness with SpO2 of 92% at room air. The left lung lavage was done after a week (Figure 6). Remarkable improvement was achieved after both lung lavage with no breathlessness on discharge, SpO2 of 97-98% at room air, marked improvement in Six minute walk test and PFT (Table 1). His Chest radiograph still revealed bilateral lower zone patchy opacities (Figure 7) and considering that this was his second episode of symptoms, we decided to give him GM-CSF injection subcutaneously. Yeast derived recombinant human granulocyte macrophage - colony stimulating factor (Sargramostim) at the dose of 9 mcg/kg body weight i.e 500 mcg once daily subcutaneously was given for 8 weeks following which he was assessed for a year during which he has remained symptom free with SpO2 of 99% at room air. |
Discussion
|
| Pulmonary Alveolar Proteinosis was ? rst reported in 1958 by Rosen et al as a five year series of 27 cases [1]. However it is still a rare disorder, with estimates of an incidence of one in two million people [3]. |
| Pulmonary Alveolar Proteinosis occurs due to deposition of amorphous lipoproteinaceous material in the alveoli. This mainly results from primary or acquired form of macrophage dysfunction that results in abnormal processing of surfactant. Over a course of time, phospholipids and surfactant apoproteins accumulate in the alveoli. There are essentially three types of PAP-congenital, acquired and secondary. In the congenital form there are mutations in the genes encoding for surfactant protein B or C or the beta chain of the receptor for granulocyte colony stimulating factor that leads to this condition [3]. In the secondary form there may be a functional impairment of macrophages caused due to immunosuppressive agents, exposure to toxic fumes or inorganic dusts like silica and cotton, hematological cancers and infections due to Nocardiaspecies, Pneumocystis jerovici, Mycobacterium tuberculosis and Mycobacterium avium-intercellulare, Cytomegalovirus and Cryptococcus etc. In an acquired type of PAP, usually there is a readily identifiable cause, such as occupational dust exposure, atypical infection, hematologic malignancy, or allogenic bone marrow transplantation. In these cases there is usually a subacute presentation with a gradual onset of symptoms [6]. |
| Approximately 33% of patients are asymptomatic at presentation, whereas others present with a myriad of symptoms, including dyspnea, dry cough, fever malaise, and respiratory failure. The physical manifestations of PAP are quite nonspecific, and pulmonary function test results are consistent with a restrictive defect [7]. Whole Lung Lavage is the gold standard treatment, which results in significant symptomatic and radiographic improvement in patients with PAP. The indications of whole lung lavage include pathologic diagnosis of PAP obtained by either transbronchial lung biopsy or open lung biopsy, severe dyspnea or hypoxemia [1]. |
| Contraindications of whole lung lavage are very few which includes blood dyscrasias, anesthetic risks and cardiopulmonary instability [1,3]. Whole lung lavage however is generally well tolerated. Low oxygen saturations (percentage in high 70s and 80s) are not uncommon early in the procedure; however, they generally improve throughout the case without any other intervention. |
| The amount of fluid that is required by lavage is dictated by change of colour from dense, opaque and cloudy to clear fluid. Pneumothorax, pleural effusion, and hydropneumothorax and other complications can be avoided by meticulous charting of the infused saline solution and output, and by taking care not to allow instilled fluid to exceed the fluid drained by more than a few hundred millimeters in consecutive lavages. Care should be taken so that spillage does not occur into the contralateral (ventilated) lung [1,3]. |
| In the majority of reported cases, a significant clinical improvement was reported following whole lung lavage for PAP. Approximately 10-15% of patients will have a relapsing course, requiring repeat procedures [1,5]. Replacement of granulocyte macrophage colony stimulating factor administered by subcutaneous injection has resulted in an increase in macrophage production, a reduction in the risk of opportunistic infections, and a reversal of the defect leading to surfactant accumulation within the alveoli and may be used in refractory cases [4,5,8] We also tried the use of daily GM-CSF in our patient on discharge but could give him only for 8 weeks due to financial constraints. At 14 months follow up he is asymptomatic with SpO2 98% at room air and showed no radiological deterioration (Table 1). |
| A point to note is that serum or BAL levels of GM-CSF could not be done due to unavailability of the relevant kit. Detailed history taking revealed no specific exposure and the onset of this disease is well into his adulthood which rules out both secondary and congenital PAP. Thus considering this case to be that of Idiopathic PAP, we had resorted to WLL. |
| This case report highlights the importance of suspecting and diagnosing a rare disorder like PAP clinically and treating it with the use of an invasive and cumbersome procedure like whole lung lavage for treatment under general anesthesia. The additional use of GM-CSF injection [though for only 8 weeks as opposed to 3-6 months in most case reports] has kept the patient symptom free for over a year. Moreover the importance of detailed history taking and clinical assessment has also been reiterated. |
Statement
|
| The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work. |
Source of support
|
| None |
Conflicts of Interest
|
| None |
Tables at a glance
|
 |
| Table 1 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
|
References
|
- Rosen SH, Castleman B, Liebow AA (1958)Pulmonary alveolar proteinosis. N Engl J Med258:1123-1142.
- Michaud G, Reddy C, ErnstA (2009)Whole-Lung Lavage for Pulmonary Alveolar Proteinosis. Chest 136:1678-1681.
- Seymour JF, Presneill JJ (2002)Pulmonary alveolar proteinosis: Progress in the first 44 years. Am J RespirCrit Care Med166:215-235.
- Sawai T, Umeyama Y, Yoshioka S, Matsuo N, Suyama N, et al. (2014)Autoimmune pulmonary alveolar proteinosis co-existing with breast cancer: a case report. Journal of medical case reports 8: 279.
- Ramirez J, Harlan WR (1968)Pulmonary alveolar proteinosis: nature and origin of alveolar lipid. The American journal of medicine 45: 502-512.
- Goldstein LS, Kavuru MS, Curtis-McCarthy P, Christie HA, Farver C, et al. (1998) Pulmonary alveolar proteinosis: Clinical features and outcomes. Chest114:1357-1362.
- Kitamura T, Tanaka N, Watanabe J (1999) Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med190:875-880.
- Huffman JA, Hull WM, DranoV G (1996) Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-de?cient mice. J Clin Invest 97:649-655.
- Reed JA, Whitsett JA (1998) Granulocyte macrophage colony stimulating factor and pulmonary homeostasis. ProcAssoc Am Physician 110:321-332.
- Ben-Dov I,Segel MJ (2014)Autoimmune pulmonary alveolar proteinosis: Clinical course and diagnostic criteria. Autoimmunity reviews 13: 513-517.
- Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C (2000) Pulmonary alveolar proteinosis: Clinical aspects and current concepts on pathogenesis. Thorax55:67-77.
- Kavuru MS, Sullivan EJ, Piccin R, Thomassen MJ, Stoller JK (2000) Exogenous granulocyte-macrophage colony-stimulating factor administration for pulmonary alveolar proteinosis. Am J RespirCrit Care Med161:1143-1148.
- Wylam ME, Ten RM, Katzmann JA, Clawson M, Prakash UBS, et al. (2000) Aerosolized GM-CSF improves pulmonary function in idiopathic pulmonary alveolar proteinosis [abstract]. Am J RespirCrit Care Med 161:A889.
- Kazkaz H, Isenberg D (2004) Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. CurrOpinPharmacol4:398-402.
- Kosacka M, Dyla T, Jankowska R (2004) Alveolar proteinosis after professional exposure to cotton and linen dust, successfully treated with whole lung lavage - a case report. PneumonolAlergol Pol 72:217-220.
- Seymour JF, Presneill JJ,Schoch OD,Downie GH,Moore PE,et al. (2001) Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J RespirCrit Care Med163:524-531.
- Bansal A,Sikri V (2013)A case of pulmonary alveolar proteinosis treated with whole lung lavage. Indian J Crit Care Med 17:314-317.
- Khan A, Agarwal R (2011)Pulmonary alveolar proteinosis. Respir Care 56:1016-1028.
|








