Robotic-Assisted Distal Pancreatectomy for a Pancreatic Desmoid Tumor: A Case Report and Review of the Literature
George Younan, Kiyoko Oshima, Darren D Ballard and Kathleen K Christians
DOI10.21767/2471-8041.100042
George Younan1, Kiyoko Oshima2, Darren D Ballard3 and Kathleen K Christians1*
1Department of Surgery, Division of Surgical Oncology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
2Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
3Division of Gastroenterology and Hepatology, Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
- *Corresponding Author:
- Kathleen K. Christians
Department of Surgery
Division of Surgical Oncology
Medical College of Wisconsin
9200 W Wisconsin Ave, Milwaukee
Wisconsin, 53226, USA
Tel: 414-805-9720
Fax: 414-805-5934
E-mail: kchristi@mcw.edu
Received date: March 10, 2016; Accepted date: March 26, 2017; Published date: March 28, 2017
Citation: Younan G, Oshima K, Ballard DD, et al. Robotic-Assisted Distal Pancreatectomy for A Pancreatic Desmoid Tumor: A Case Report and Review of the Literature. Med Cas Rep. 2017, 3:1. doi: 10.21767/2471-8041.100042
Abstract
Introduction: Desmoid tumors are rare, indolent tumors of mesenchymal origin, with a potential for local invasion and adjacent organ involvement, without a metastatic potential. Pancreatic desmoid tumors are defined as tumors involving the pancreas parenchyma, sparing adjacent organs, and requiring a pancreatectomy for adequate surgical resection.
Case presentation: We report a case of a lesser sac heterogeneous solid tumor involving the pancreatic tail in a 69-year-old man without a known genetic predisposition. Treatment consisted of a robotic-assisted distal pancreatectomy and splenectomy due to the intimate anatomical association of the mass to the pancreatic parenchyma and splenic vessels. The mass was found to be a desmoid-type fibromatosis on pathological assessment.
Discussion: Surgical resection with adequate margins remains the gold curative standard for desmoid-type fibromatosis tumors of the abdomen and retroperitoneum. Pancreatic desmoid tumors tend to be sporadic, occur equally among genders, most commonly involving the distal body and tail of the pancreas, requiring a distal pancreatectomy for adequate surgical margins.
Conclusion: We report in this article the twenty first case of a pancreatic desmoid tumor in the literature and the first report of a robotic-assisted resection of this tumor.
Keywords
Pancreatectomy; Desmoid; Fibromatosis; Robotics
Introduction
Desmoid tumors (DT) are locally aggressive soft tissue neoplasms of mesenchymal origin [1]. Also, known as aggressive fibromatoses, desmoid-type fibromatoses or musculo-aponeurotic fibromatoses; they are indolent, slow growing tumors that tend to cause aggressive local invasion of surrounding organs but do not metastasize [2]. Histologically, DTs are characterized by a monoclonal proliferation of fibroblasts and myofibroblasts arising from any fibrous tissue in the body [3]. DTs are classified by their location, as extraabdominal or abdominal.4 Extra-abdominal tumors occur most commonly in the pelvis, chest wall or shoulder. Abdominal tumors can be located superficially in the abdominal wall or deep within the abdominal cavity arising from the retroperitoneum or the mesentery [4]. DTs may also be classified as sporadic or familial; the sporadic form is the most common (> 90% of the cases) and usually involves extraabdominal locations whereas the familial form occurs in 10% to 15% of Familial Adenomatous Polyposis (FAP) patients [5,6]. Gardner syndrome is defined as the concomitant occurrence of DTs in FAP patients, both familial syndromes resulting from an inactivating mutation of the APC gene [7]. Most of the FAPassociated desmoids are intra-abdominal in location; they infiltrate the small bowel mesentery and cause local symptoms of obstruction and compression of surrounding structures [4]. In FAP patients who have had a prophylactic colectomy to prevent colon cancer, desmoid tumors of the mesentery are the most common cause of morbidity [8]. The etiology of DTs remains controversial. A strong correlation with the hormonal status of patients has been reported in the literature; tumors tend to be more common in women, especially in their reproductive years and typically regress after menopause or due to the anti-estrogen activity of tamoxifen [9]. Tissue trauma has been also implicated in the occurrence of fibromatosis; tumors tend to grow in areas close to postoperative scars, or after colectomy in FAP patients [10]. Pancreatic DTs are extremely rare and are defined as pancreatic due to their intimate association with pancreatic parenchyma in the absence of involvement of surrounding organs and necessitate pancreatectomy for their removal [11].
We report a case of a sporadic, non-traumatic, desmoid-type fibromatosis pancreatic tumor treated by robotic-assisted distal pancreatectomy and splenectomy and review the literature for this very rare condition.
Case Presentation
A 69-year-old man was referred to the surgical oncology clinic with a left upper quadrant solid mass of unknown etiology in a setting of abdominal discomfort, nausea and a history of ocular melanoma. The patient was diagnosed with choroidal ocular melanoma two years prior to his current complaints and was treated with radiation therapy, however it recurred and he was offered additional radiation therapy.
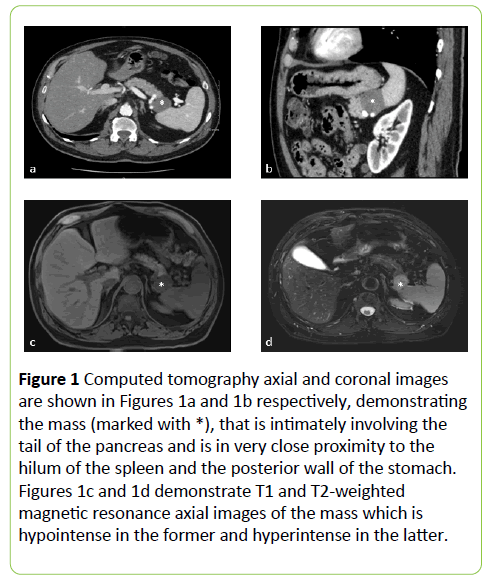
At the time of recurrence, a staging computed tomography scan showed a 3.5 x 2.5 cm well circumscribed solid mass in the left upper quadrant, potentially involving the posterior wall of stomach, distal body and tail of the pancreas and the splenic hilum (Figures 1a and 1b).
Figure 1: Computed tomography axial and coronal images are shown in Figures 1a and 1b respectively, demonstrating the mass (marked with *), that is intimately involving the tail of the pancreas and is in very close proximity to the hilum of the spleen and the posterior wall of the stomach. Figures 1c and 1d demonstrate T1 and T2-weighted magnetic resonance axial images of the mass which is hypointense in the former and hyperintense in the latter.
By magnetic resonance imaging, the mass had homogenous enhancement on delayed images and was hypointense on T1- weighted images and hyperintense on T2-weighted images. (Figures 1c and 1d) The differential diagnosis from an imaging perspective was an exophytic pancreatic acinar tumor, a gastrointestinal stromal tumor (GIST) of the stomach or a metastatic lesion of unknown origin but potentially related to his personal history of melanoma.
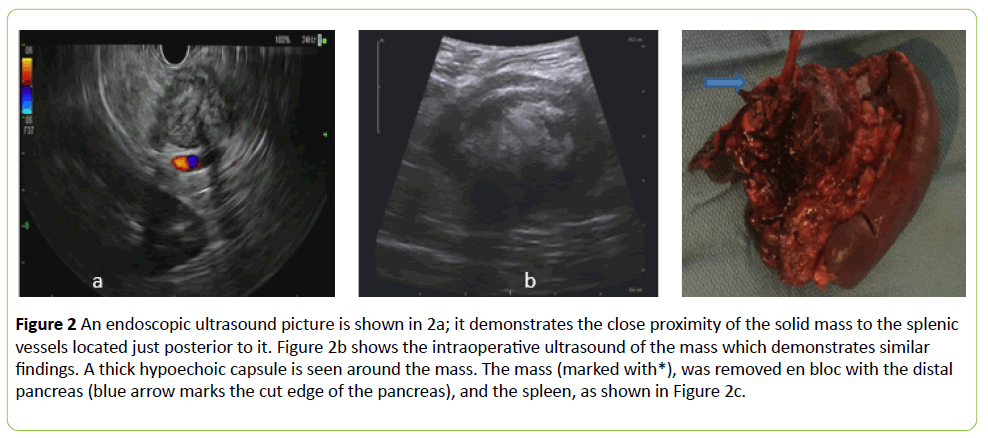
The patient was referred to gastroenterology and an endoscopic ultrasound was performed twice with fine needle aspiration (FNA) biopsy once, then a core biopsy of the tumor was also performed. (Figure 2a) The FNA contained some atypical cells and bland spindle cells that stained negative for GIST and melanoma markers (would specify).
Figure 2: An endoscopic ultrasound picture is shown in 2a; it demonstrates the close proximity of the solid mass to the splenic vessels located just posterior to it. Figure 2b shows the intraoperative ultrasound of the mass which demonstrates similar findings. A thick hypoechoic capsule is seen around the mass. The mass (marked with*), was removed en bloc with the distal pancreas (blue arrow marks the cut edge of the pancreas), and the spleen, as shown in Figure 2c.
These findings were non-diagnostic. The patient was therefore referred to our surgical oncology clinic for resection of this mass. The patient’s complaints were limited to mild abdominal discomfort and morning nausea; he denied history of pancreatic insufficiency or obstruction. He denied weight loss.
His past medical history is significant for recurrent ocular melanoma, hypertension, and hyperlipidemia. His recent colonoscopy was three years prior to his current presentation, where three tubular adenomas were endoscopically resected. He also had a craniotomy to remove a tumor from his skull as a youth; however, the final pathology is unknown. Family history is significant for colon cancer in his mother.
The patient was consented for resection of this mass, and a robotic-assisted approach was thought to be feasible in this case. The consent included the possibility of resection of surrounding structures that might have been involved including the spleen, pancreas and stomach.
The patient was then taken to the operating room and a robotic-assisted approach was used to enter the lesser sac and examine the mass. An intraoperative ultrasound was used to assess the relationship of the mass to the pancreas and surrounding vasculature (Figure 2b).
It became clear early in the dissection that this mass did not involve the stomach; however, it was fused to the pancreas and abutted the spleen necessitating a distal pancreatectomy and splenectomy. The lesion was inspected on the back table.
It was hard and was inseparable from the pancreas (Figure 2c). It was bisected which revealed an encapsulated mass with white, thick stroma (Figure 3a). This was sent en bloc for pathological assessment, the patient’s postoperative course was uncomplicated. Final pathology revealed a five centimeter desmoid-type fibromatosis, diffusely positive for beta-catenin by immunohistochemistry, and negative for smooth muscle actin, desmin, CD34, S100, AE1/AE3 and BCL-2 (Figure 3).
Figure 3: A gross pictures obtained by pathology shows the mass bi-valved, demonstrating its thick gray, granular nature in Figure 3a. Figure 3b is a low magnification picture of a cross section of the mass in the pancreas; a thick fibrous capsule connects the pancreas tissue (left, deep purple) to the mass (right, pink). Figure 3c shows a Hematoxylin and eosin stain of the mass under low magnification (x40); the fibromatosis is composed of fascicles of slender fibroblasts separated by collagen. Figure 3d demonstrates the immunohistochemical stain for beta-catenin (x100). Betacatenin stain shows positive nuclear stain in the tumor, and confirms the diagnosis of fibromatosis (Arrows).
Discussion
Primary mesenchymal tumors of the pancreas are a very rare pathological entity; most mesenchymal tumors involving the pancreas are thought to arise from surrounding structures that then involve the pancreas [11]. The most common cells in the pancreas are acinar and ductal cells, followed by islet cells. Stromal cells are usually scant in the normal pancreas.
Therefore, the most common tumors of the pancreas are ductal adenocarcinomas and neuroendocrine tumors; pancreatic DTs are very rare mesenchymal tumors. DTs most commonly invade the body or tail of the pancreas, cause local compression and invade surrounding structures of the lesser sac [7]. DTs were first described by McFarlane in 1832 as a pathological entity resulting from fibroblast proliferation [12]. The actual term “desmoid” was used by Muller in 1838 who described their histologic consistency and appearance as the Greek word “desmos” which means tendon or ligament-like [4]. DTs were discovered to be associated with FAP syndrome in 1923 by Nichols [13]. The first pancreatic DT was described by Wilson from Australia in 1956, and was believed to be a pancreatic pseudocyst [14].
DTs in general are rare tumors, accounting for 0.03% of all tumors and only 3% of soft tissue tumors [9]. The incidence in the general population is 2-4 per million per year [15]. Intraabdominal forms of DTs occur in only 8% of the cases and pancreatic DTs are exceedingly rare with only twenty cases reported in the English literature to date [7,9,16]. Preoperative diagnosis of pancreatic DTs is usually difficult as they tend to resemble other solid or cystic tumors of the pancreas and other organs around the lesser sac of the abdomen. They are slow growing and usually present with abdominal pain as was the case with our patient, or are incidentally discovered during imaging done for other reasons [16]. While most DTs are slow growing, non-metastatic, locally infiltrating and either compress or obstruct surrounding structures and are amenable to resection, some tumors are fast growing and cause bowel perforation, bleeding and bowel ischemia by invading the mesenteric blood supply [17,18]. This tumor behavior has been historically labelled as “locally malignant” [4]. Church reported a 10% spontaneous resolution rate of DTs, and a 10% rapid progression rate, while 50% remain stable on follow up and 30% have cycles of progression and resolution [4,19]. Little is known about the natural history of pancreatic DTs; most (give the n) were resected and the two deaths reported in the literature were not treated with surgery.7 Pancreatic DTs are hard to diagnose preoperatively. The majority are sporadic, thus there is usually less suspicion of a desmoid as is the case in FAP patients. Biopsy of DTs is usually non-diagnostic as fibrous tissue is sampled and thought to be a sampling error. Ultrasound examination of DTs reveals well circumscribed tumors with variable echogenicity while computed tomography (CT) scans show the relative association of the high attenuating tumor to the surrounding structures. Magnetic resonance imaging (MRI) scans show DTs having low signal intensity on T1-weighted images and variable signal intensity on T2-weighted images [20].
Surgery, once considered as the cornerstone treatment for DTs, is now being integrated as a component of a multimodality approach in multidisciplinary programs treating this entity. In recent years, systemic therapy has proven its positive effect in the treatment of DTs [21]. Additionally recent data has proven the safety of observation of abdominal desmoids as first line strategy, owing to the slow growing nature of this disease and the reasonable rates of spontaneous regression or prolonged stability [2] There is not enough data to extrapolate these findings to pancreatic DTs due to the inability to offer systemic therapy in the absence of adequate tissue diagnosis and/or the low level of comfort of patients and their treating oncologists to observe a solid pancreatic lesion. The National Comprehensive Cancer Network (NCCN) guidelines suggest observation of these tumors unless symptomatic or causing impairment of organ function, therapy then is chosen based on the location and potential resulting morbidity of the procedure [22,23]. There has been no consensus on the surgical margin requirements when performing desmoid resections, some studies have clearly shown that an R1 margin is an independent risk factor for local recurrence while other studies showed no difference between R0 and R1 resections if patients are closely followed [24-26].Thus, there is an agreement on accepting R1 resections if obtaining an R0 margin would pose significant morbidity to the patient.
In the case of locally advanced, surgically unresectable tumors, a few systemic therapies have been implicated, however radiation therapy is generally not recommended for intra-abdominal and retroperitoneal desmoid neoplasms. NCCN guidelines include anti-inflammatory agents (celecoxib, sulindac), anti-hormonal agents (tamoxifen, interferons), tyrosine kinase inhibitors (imatinib, sorafenib) and chemotherapy agents (doxorubicin, vinblastine, methotrexate), to be used in cases of advanced, surgically unresectable disease or in patients that are not surgical candidates due to co-morbid conditions [27-33].
Our case is the twenty-first case of a pancreatic DT reported in the English literature; twenty previous cases have been identified and are summarized in Table 1 [7,9,11,16,34-45]. DTs occurring in the pancreas, unlike other intra-abdominal DTs, are most commonly sporadic with only one reported pancreatic tail solid/cystic DT reported to be associated with FAP.40 Tumors were equally distributed between genders (12 males and 9 females). Fourteen percent of the cases had a previous history of a pancreatectomy, indicating the relationship between DTs and previous trauma/surgery to the area [7,37,38,41]. Sixty-two percent of the tumors were found to be in the body/tail of the pancreas, necessitating a distal pancreatectomy in 75% of the cases who underwent surgical resection. Recurrence rate was reported to be 18.2% by Tsakumoto et al. [7]. A very interesting observation can be made about these tumors; the majority was assigned a different diagnosis pre-operatively, pointing towards their rarity and the difficulty in making the correct diagnosis.
| Study | Year | Location | Gender | Age | FAP | Size (cm) | Consistency | Surgery |
|---|---|---|---|---|---|---|---|---|
| Roggli at al. [35] | 1980 | Whole | M | 4m | No | NA | Solid | None |
| Ure et al.[36] | 1988 | Head/Body | F | 2m | No | 4 | Solid | EN |
| Bruce et al.[37] | 1996 | Tail | M | 38 | No | 5 | Solid | DP |
| Sedivy et al.[38] | 2002 | Head | F | 68 | No | 1.5 | Solid | None |
| Nursal et al.[39] | 2003 | Tail | F | 25 | No | 8.5 | Solid | None |
| Nursal et al.[39] | 2003 | Tail | M | 39 | No | 7.5 | Solid | None |
| Pho et al.[40] | 2005 | Tail | M | 17 | Yes | 4 | Mixed | DP |
| Weiss et al.[41] | 2006 | Tail | M | 63 | No | 6.5 | Solid | DPS |
| Amiot et al.[42] | 2008 | Tail | F | 51 | No | 6 | Mixed | DPS |
| Polistina et al.43 | 2010 | Tail | M | 68 | No | 5 | Mixed | DP |
| Rao et al.[16] | 2013 | Tail | M | 11 | No | 10 | Mixed | DPS |
| Xu at al.[44] | 2013 | Body | M | 17 | No | 8.6 | Mixed | CP |
| Kim et al.[11] | 2014 | Head | F | 33 | No | 2.3 | Solid | PD |
| Kim et al.[11] | 2014 | Head | F | 49 | No | 1.5 | Solid | PD |
| Kim et al.[11] | 2014 | Tail | M | 72 | No | 5 | Solid | DP |
| Kim et al.[11] | 2014 | Head | M | 40 | No | 8 | Solid | None |
| Gerleman et al.[45] | 2015 | Tail | F | 63 | No | 5.8 | Solid | DP |
| Mourra et al.[34] | 2015 | Tail | F | 20 | No | 7.5 | Cystic | DPS |
| Slowik-Moczydlowsa et al.[9] | 2015 | Tail | M | 13 | No | 10 | Cystic | DPS |
| Tsukamoto et al.[7] | 2016 | Tail | F | 75 | No | 8 | Solid | DPS |
| Younan et al. | 2016 | Tail | M | 69 | No | 5 | Solid | DPS |
Table 1: Twenty previous cases of pancreatic DT reported earlier in the English literature.
Conclusion
Pancreatic DTs are very rare mesenchymal tumors of the pancreas. Unlike other intra-abdominal DTs, they are not associated with a familial syndrome and tend to be sporadic. They most commonly affect the distal body and the tail of the pancreas necessitating a pancreatectomy to obtain adequate oncological margins. Although surgical resection has been the standard of care for these tumors, observation and systemic therapy are gaining success as a first line approach to pancreatic DTs in a multidisciplinary setting, assuming a correct tissue diagnosis can be obtained.
References
- Wu C, Amini-Nik S, Nadesan P, Stanford WL, Alman BA (2010) Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells. Cancer res 70: 7690-7698.
- Burtenshaw SM, Cannell AJ, McAlister ED, Siddique S, Kandel R, et al. (2016) Toward observation as first-line management in abdominal desmoid tumors. Ann SurgOncol 23: 2212-2219.
- Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ (1997) Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. DiagnMolPathol 6 :98-101.
- Sakorafas GH, Nissotakis C, Peros G (2007) Abdominal desmoid tumors. Surgoncol 16: 131-142.
- Gurbuz AK, Giardiello FM, Petersen GM, Krush AJ, Offerhaus GJ, et al. (1994) Desmoidtumours in familial adenomatous polyposis. Gut 35: 377-381.
- Brueckl WM, Ballhausen WG, Fortsch T, Günther K, Fiedler W, et al. (2005) Genetic testing for germline mutations of the APC gene in patients with apparently sporadic desmoid tumors but a family history of colorectal carcinoma. Diseases of the colon and rectum 48: 1275-1281.
- Tsukamoto Y, Imakita M, Nishitani A, Ito T, Izukura M, et al. (2016) Pancreatic desmoid-type fibromatosis with beta-catenin gene mutation-Report of a case and review of the literature. Pathology, research and practice 212: 484-489.
- Nugent KP, Spigelman AD, Phillips RK (1993) Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Diseases of the colon and rectum. 36: 1059-1062.
- Slowik-Moczydlowska Z, Rogulski R, Piotrowska A, Maldyk J, Kluge P, et al. (2015) Desmoid tumor of the pancreas: A case report. Journal of medical case reports 9: 104.
- Cohen S, Ad-El D, Benjaminov O, Gutman H (2008) Post-traumatic soft tissue tumors: case report and review of the literature a propos a post-traumatic paraspinaldesmoid tumor. World journal of surgical oncology 6: 28.
- Kim JY, Song JS, Park H, Byun JH, Song KB, et al. (2014) Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas. 43: 959-968.
- MacFarlane J (1832) Clinical reports on the surgical practice of Glasgow Royal Infirmary. D. Robertson,Glasgow p:63-66.
- Nichols RW (1923) Desmoid tumors: A report of thirty-one cases. Arch Surg 7: 227-236.
- Wilson E (1956) Desmoid tumor resembling a pseudo-pancreatic cyst in a male. BMJ 2: 982-983.
- Papagelopoulos PJ, Mavrogenis AF, Mitsiokapa EA, Papaparaskeva KT, Galanis EC, et al. (2006) Current trends in the management of extra-abdominal desmoidtumours. WJSO 4: 21.
- Rao RN, Agarwal P, Rai P, Kumar B (2013) Isolated desmoid tumor of pancreatic tail with cyst formation diagnosed by beta-catenin immunostaining: a rare case report with review of literature. JOP 14: 296-301.
- Xhaja X, Church J (2013) Small bowel obstruction in patients with familial adenomatous polyposis related desmoid disease. Colorectal Disease 15: 1489-1492.
- Quintini C, Ward G, Shatnawei A, Xhaja X, Hashimoto K, et al. (2012) Mortality of intra-abdominal desmoid tumors in patients with familial adenomatous polyposis: a single center review of 154 patients. Ann surg 255: 511-516.
- Church J, Lynch C, Neary P, LaGuardia L, Elayi E (2008) A desmoid tumor-staging system separates patients with intra-abdominal, familial adenomatous polyposis-associated desmoid disease by behavior and prognosis. Diseases of the colon and rectum. 51: 897-901.
- Overhaus M, Decker P, Fischer HP, Textor HJ, Hirner A (2003) Desmoid tumors of the abdominal wall: A case report. WJSO 1: 1-7.
- Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, et al. (2007) Optimizing treatment of desmoid tumors. J clinoncol 25: 1785-1791.
- VonMehren M, Randall RL, Benjamin RS, Boles S, Bui MM, et al. (2016) Soft tissue sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J NatlComprCancNetw 14: 758-786.
- Fiore M, Rimareix F, Mariani L, Domont J, Collini P, et al. (2009) Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann surgoncol 16: 2587-2593.
- Cates JM, Stricker TP (2014) Surgical resection margins in desmoid-type fibromatosis: A critical reassessment. Am J Pathol 38: 1707-1714.
- Crago AM, Denton B, Salas S, Dufresne A, Mezhir JJ, et al. (2013) A prognostic nomogram for prediction of recurrence in desmoidfibromatosis. Ann Surg 258: 347-353.
- Salas S, Dufresne A, Bui B, Blay JY, Terrier P, et al. (2011) Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J ClinOncol 29: 3553-3558.
- Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G (2004) High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100: 612-620.
- Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS (2003) The pharmacological treatment of aggressive fibromatosis: A systematic review. Ann Oncol 14: 181-190.
- Leithner A, Schnack B, Katterschafka T, Wiltschke C, Amann G, et al. (2000) Treatment of extra-abdominal desmoid tumors with interferon-alpha with or without tretinoin. J SurgOncol 73: 21-25.
- Poritz LS, Blackstein M, Berk T, Gallinger S, McLeod RS, et al. (2001) Extended follow-up of patients treated with cytotoxic chemotherapy for intra-abdominal desmoid tumors. Diseases of the colon and rectum. 44: 1268-1273.
- Garbay D, Le Cesne A, Penel N, Chevreau C, Marec-Berard P, et al. (2012) Chemotherapy in patients with desmoid tumors: A study from the French Sarcoma Group (FSG). Ann Oncol 23: 182-186.
- Heinrich MC, McArthur GA, Demetri GD, Joensuu H, Bono P, et al. (2006) Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J ClinOncol 24: 1195-1203.
- Chugh R, Wathen JK, Patel SR, Maki RG, Meyers PA, et al. (2010) Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 16: 4884-4891.
- Mourra N, Ghorra C, Arrive L (2015) An unusual solid and cystic pancreatic tumor in a 20-year-old woman. Desmoidtumor: Fibromatosis. Gastroenterology 149: e5-6.
- Roggli VL, Kim HS, Hawkins E (1980) Congenital generalized fibromatosis with visceral involvement, A case report. Cancer 45: 954-960.
- Ure BM, Holschneider AM, Gharib M, Halsband H, Hinselmann D (1988) Clinical aspects, classification and prognosis of 7 cases of pediatric fibromatosis. Surgery in infancy and childhood 43: 27-30.
- Bruce JM, Bradley EL 3rd, Satchidanand SK (1996) A desmoid tumor of the pancreas. Sporadic intra-abdominal desmoids revisited. Int j pancreatol 19: 197-203.
- Sedivy R, Ba-Ssalamah A, Gnant M, Hammer J, Kloppel G (2002) Intraductal papillary-mucinous adenoma associated with unusual focal fibromatosis: A post-operative stromal nodule. VirchowsArchiv 441: 308-311.
- Nursal TZ, Abbasoglu O (2003) Sporadic hereditary pancreatic desmoid tumor: A new entity? J ClinGastroenterol 37: 186-188.
- Pho LN, Coffin CM, Burt RW (2005) Abdominal desmoid in familial adenomatous polyposis presenting as a pancreatic cystic lesion. Familial cancer 4: 135-138.
- Weiss ES, Burkart AL, Yeo CJ (2006) Fibromatosis of the remnant pancreas after pylorus-preserving pancreatico-duodenectomy. J Gastrointestinal 10: 679-688.
- Amiot A, Dokmak S, Sauvanet A, Vilgrain V, Bringuier PP, et al. (2008) Sporadic desmoid tumor. An exceptional cause of cystic pancreatic lesion. JOP 9: 339-345.
- Polistina F, Costantin G, D'Amore E, Ambrosino G (2010) Sporadic, non-trauma-related, desmoid tumor of the pancreas: A rare disease-case report and literature review. Case reports in medicine 272760.
- Xu B, Zhu LH, Wu JG, Wang XF, Matro E, et al. (2013) Pancreatic solid cystic desmoid tumor: Acase report and literature review. WJG. 19: 8793-8798.
- Gerleman R, Mortensen MB, Detlefsen S (2015) Desmoidtumor of the pancreas: Acase report and review of a rare entity. Int J SurgPathol 23: 579-584.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences