Subacute Intestinal Obstruction in a Patient of Congestive Heart Failure
Hina Muqeem* and Jamal Azmat
Department of Moalejat, Aligarh Muslim University, Uttar Pradesh, India
- *Corresponding Author:
- Hina Muqeem
Department of Moalejat, Aligarh Muslim University, Uttar Pradesh,
India,
E-mail: Hinamuqeem076@gmail.com
Received date: February 27, 2023, Manuscript No. IPMCRS-23-15961; Editor assigned date: March 01, 2023, PreQC No. IPMCRS-23-15961 (PQ); Reviewed date: March 14, 2023, QC No. IPMCRS-23-15961; Revised date: March 21, 2023, Manuscript No. IPMCRS-23-15961 (R); Published date: March 28, 2023, DOI: 10.36648/2471-8041.9.3.267
Citation: Muqeem H, Azmat J (2023) Subacute Intestinal Obstruction in a Patient of Congestive Heart Failure. Med Case Rep Vol.9 No.3: 267.
Abstract
Sub-acute intestinal obstruction is characterized by continued passage of flatus and/or faeces beyond 6-12 hrs. After onset of symptoms namely colicky abdominal pain, vomiting, and abdominal distension.
Congestive heart failure is a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood leading to insufficient blood perfusion to meet the body’s requirements. The cardinal manifestations of HF are dyspnoea and fatigue, which may limit exercise tolerance, and fluid retention. In patient of CHF, SAIO may result due to tissue anoxia which leads to low perfusion (i.e., low cardiac output), congestion (i.e., increased central venous pressure), and increased sympathetic vasoconstriction. The combination of hypoxia and local production of lipopolysaccharides by gram-negative bacteria residing in the gut lumen causes an increased paracellular permeability of the intestinal wall. Hence, CHF patients are at risk of nonocclusive bowel ischemia.
Keywords
Congestive heart failure; Sub acute intestinal obstruction; Hypoxia; Digoxin; Lipopolysaccharides
Case Report
A 59 year old male patient presented to OPD of medicine with chief complaints of constipation and lower abdominal pain and distention from last 7 days and breathlessness from last 2 years.
On history taking, he complained of not passing stool, abdominal pain and distention from last 7 days which was insidious in onset and intermittent coliky, bloating in nature. The pain was diffuse and more pronounced in infraumblical region with feeling of fullness and decreased appetite. There is no history of nausea, vomiting and fever [1-4].
There was breathlessness on exertion from last 2 year which started insidiously and progressive in nature aggravating on lying down position without any chest pain and syncope. The personal history revealed him to be a chronic smoker and a known case of post MI cardiomyopathy for which he had been taking treatment since last 2 year which consists of the combination of Digoxin, Beta Antagonist ACE inhibitors, Diuretic, Antiplatelet and Statins.
On general examination, the patient was dyspnoeic, bilateral pedal pitting edema was present.
BP-130/90 mmHg, pulse 112 bpm with normal rhythm and volume, SpO2 was 97% (ambient air) temperature was 98 F, respiratory rate was 20/minute and JVP was not raised with no lymphadenopathy and clubbing.
Clinically the sign of hypotention was absent except tachycardia which may be due to CHF. There was no sign of dehydration like mottled skin, sunken eyes or decreased urinary output [5].
Per abdominal examination
Inspection: Tense abdomen with no visible peristalsis, pulsation, scar.
Palpation: Mild tenderness present all over the abdomen without guarding and rigidity which was marked on hepatic area with positive hepatojugular reflux.
On auscultation: Bowel sound were diminished.
Per rectal examination revealed that rectum was partially loaded with stool. Respiratory system examination revealed chest was B/L symmetrical in shape, trachea was midline on inspection. On palpation, the trachea was centrally placed, vocal fremitus was normal and local tenderness was absent. Percussion showed resonant note all over the chest. Auscultation revealed B/L fine basal crepts. Vocal resonance was normal. Other systemic examination was found to be normal [6].
On the basis of history and physical examination, the investigations done are as follows
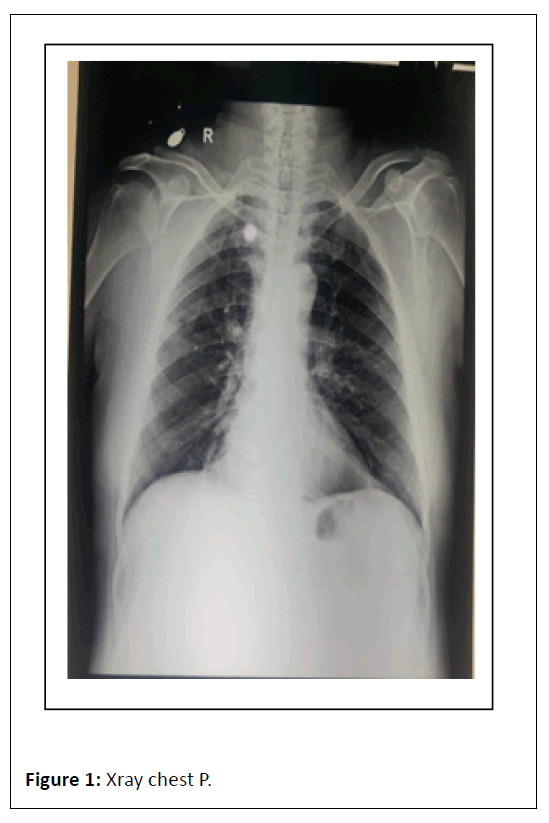
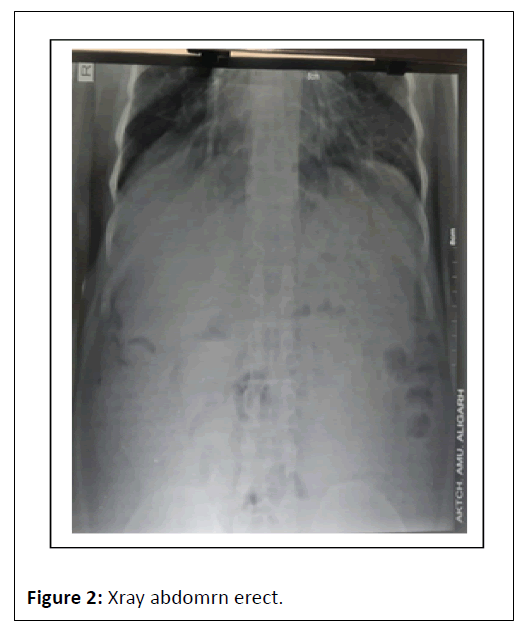
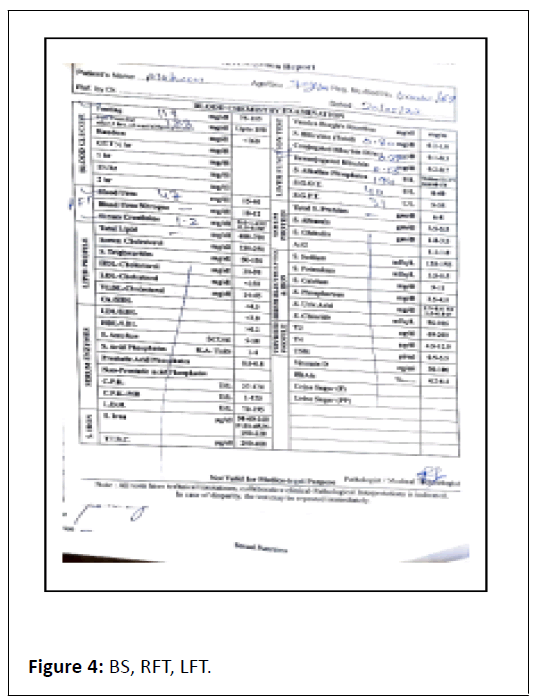
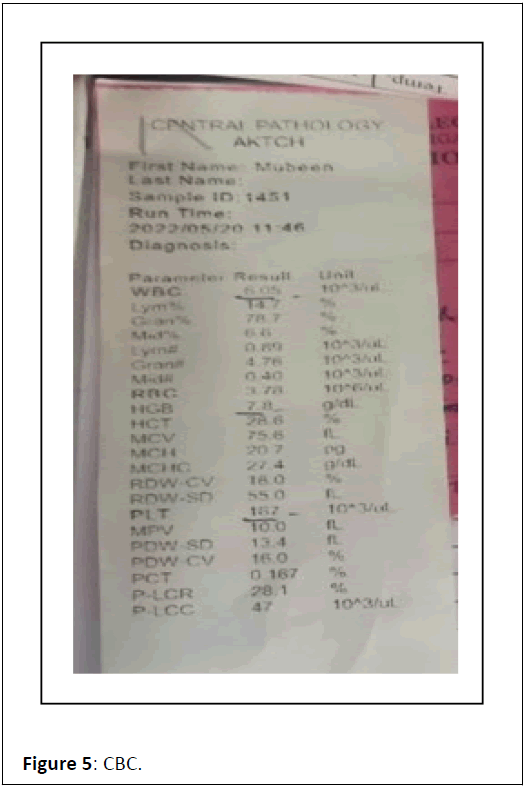
ECG showed Q wave in anterior chest leads with sinus tachycardia. Complete blood count showed TLC-6.05/103/ul, Hb-7.8 gm/dl, X-ray chest P. A view film showed cardiomegaly, prominent bronchovascular markings and few calcification in hilar area , X-ray abdomen erect revealed multiple air-fluid level in step ladder distribution which confirmed intestinal obstruction, x-ray findings were further confirmed by ultrasound abdomen which was suggestive of dilated bowel loops and showing inefficient bowel peristaltic movements confirming SAIO, Urine RM, RFT and LFT were WNL. On confirming the diagnosis of SAIO we started management as follows Figures 1-6.
Oral intake of the patient was restricted and intravenous fluid started. Two unit normal saline (0.9%) with 10 ml KCL, 2 unit RL, 1 unit DNS (5%) I.V over 24 hours to replace fluid loss and correction of electrolyte imbalance started after confirming adequate renal function. Antibiotics were given to treat intestinal overgrowth of bacteria and translocation across the bowel wall [7-10].
Injection ceftriaxone 2 gm I.V BD, injection metronidazole 400 I.V TDS, injection pantaprazole 40 mg I.V OD, injection metoclopramide I.V BD, injection frusamide (20) I.V OD along with the treatment of CHF.
The treatment was continued for 7 days and the patient was discharged on treatment of CHF.
Results and Discussion
CHF has an impact on the musculoskeletal, neuroendocrine, metabolic and immunological systems in addition to the heart and blood circulation. Alterations in intestinal morphology, permeability, hypoperfusion and absorption capacity are all linked to CHF. Increased intestinal permeability in CHF patients may result in bacterial translocation and endotoxin production, according to the endotoxin hypothesis. An enhanced endotoxin challenge may activate the immune system, resulting in higher TNF-production. The absence of functional tests that can recognize and categories the severity of gastrointestinal ischaemia is the greatest obstacle to evaluating whether CHF patients exhibit intestinal hypo perfusion. In the microcirculation, arterioles, capillaries and venules form a counter current system that resembles the vasa recta of the renal medulla in an unusual way. As a result, the intestinal villus can provide the interstitial concentration gradient that is required for continuous nutrient absorption, with the highest osmolality at its tip. This mechanism has the problem that arteriolar oxygen short circuit to venules before it reaches the villus tip, which makes this area particularly vulnerable to anoxic injury. Patients with CHF are at risk for non-occlusive intestinal ischemia due to inadequate perfusion (low cardiac output), congestion (increased central venous pressure) and increased sympathetic vasoconstriction. Importantly, the paracellular permeability of the intestinal wall is increased by the interaction of local lipopolysaccharide synthesis by gram-negative bacteria found in the gut lumen and hypoxia. Arterioles, capillaries and their function are all significantly altered in CHF, while venules in severe intestinal conditions with cardiac cachexia have a distinctive organisation. Concomitant uraemia brought on by renal failure also changes the bacterial composition of the gut and may enhance intestinal permeability. The fermentation processes in the gut that result in protein-bound uremic toxins that are ineffectively removed from the blood in cases of renal dysfunction have been shown to be caused by microbiota. Moreover, lipopolysaccharide in the circulation triggers systemic inflammation and cytokine generation (i.e., tumor necrosis factor-x, interleukin-6, which results in depression of excitation contraction coupling, decreased peak velocity of cardio myocyte shortening, disturbed mitochondrial respiration and impaired substrate metabolism in cardiomyocytes.
Conclusion
Therefore, compared to healthy individuals, CHF patients may frequently experience ischaemic stress in the gut, which may lead to the development of a compromised gut barrier function, bacterial translocation, and endotoxin uptake into the circulation, ultimately resulting in sub-acute intestinal obstruction. The abdomen in CHF is still generally understudied despite the fact that patients regularly present with abdominal symptoms, particularly when they have obvious signs and symptoms of congestion. Cardio-abdominal-renal interactions most likely have a significant influence in the development of prolonged systemic congestion despite appropriately dosed diuretic therapy, despite not always being simple to assess at the bedside. New diagnostic and treatment approaches for CHF may be possible with a better knowledge of these interactions provided by the splanchnic organs, blood vessels and microcirculation's capacitance function.
References
- Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, et al. (2013) Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 62: 485-495.
[Crossref], [Google Scholar], [Indexed]
- Cui X, Ye L, Li J, Jin L, Wang W, et al. (2018) Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep 8: 635.
[Crossref], [Google Scholar], [Indexed]
- Wang Z, Cai Z, Ferrari MW, Liu Y, Li C, et al. (2021) The correlation between gut microbiota and serum metabolomic in elderly patients with chronic heart failure. Mediators Inflamm 2021: 5587428.
[Crossref], [Google Scholar], [Indexed]
- Krack A, Richartz BM, Gastmann A, Greim K, Lotze U, et al. (2004) Studies on intragastric PCO2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur J Heart Fail 6: 403–407.
[Crossref], [Google Scholar], [Indexed]
- Fauci AS, Jameson JL, Kasper DL, Hauser SL, Longo DL, et al. (2018) Harrison's princioles of internal medicine. (20th ed), Mc graw hill education, NY, USA: 2294-2298.
- Kundu AK (2018) Bedside clinics in Medicne. (6th ed), Academic publishers 5A, Bhawani Dutta lane, Kolkata, India: 131.
- Ojha A, Jalaj A, Tiwari S, Mujalde V, Prasheel V (2014) Diagnosis and management of subacute intestinal obstruction: A prospective study. Journal of Evolution of Med and Dent Sci 3: 10-15
- George CF (1980) Drugs causing intestinal obstruction: A review. J R Soc Med 73: 200-204.
[Crossref], [Google Scholar], [Indexed]
- Das S (2010) A concise textbook of surgery. (6th ed), Old Mayors ‘Court, Kolkata, India: 880-886.
- Tripathi KD (2016) Essentials of medical pharmacology. (7th ed), Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, Inida: 218-233.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences