Stiff-Person Syndrome: A Rare Neurological Disorder
Amit Mandal*
Department of General Medicine, Christian Medical College, Vellore, India
- *Corresponding Author:
- Amit Mandal
Department of General Medicine,
Christian Medical College, Vellore,
India,
E-mail: amit.mandal78@yahoo.co.in
Received date: March 31, 2023, Manuscript No. IPMCRS-23-16317; Editor assigned date: April 03, 2023, PreQC No. IPMCRS-23-16317 (PQ); Reviewed date: April 13, 2023, QC No. IPMCRS-23-16317; Revised date: April 24, 2023; Manuscript No. IPMCRS-23-16317 (R); Published date: April 28, 2023, DOI: 10.36648/2471-8041.9.3.270
Citation: Mandal A (2023) Stiff-Person Syndrome: A Rare Neurological Disorder. Med Case Rep Vol.9 No.3: 270.
Abstract
The Stiff-person syndrome is an uncommon disorder characterized by progressive rigidity, muscle stiffness and spasm involving the axial muscles, resulting in severe impairment of ambulation. We present the case of a 49 years old gentleman with recent onset of progressive asymmetric spastic ataxia, subsequently diagnosed with Stiff-person syndrome.
Keywords
Stiff-person syndrome; Anti-Glutamic Acid Decarboxylase (GAD) antibodies; Type 1 diabetes mellitus
Introduction
The Stiff-Person Syndrome (SPS) is a rare disorder, characterized by progressive fluctuating muscular rigidity and spasms. It is triggered by increased muscle activity due to reduced inhibition by the central nervous system resulting from the blockade of Glutamic Acid Decarboxylase (GAD), an enzyme crucial for maintaining inhibitory pathways. Stiff-person syndrome is often associated with Type 1 Diabetes Mellitus (T1DM), as well as other autoimmune disorders. It may also occur as a paraneoplastic disorder.
Case Presentation
A 49 years old married gentleman, a teacher by profession, presented with pain and stiffness of the right lower limb and progressive reduction in velocity while walking short steps for a month with slurring of speech for a week. The patient was apparently functioning well before a month, following which he presented with symptoms characterized by feeling of giddiness and a sense of being pushed while walking. On consultation he was found to have high blood glucose and was initiated on oral therapy. A week later he developed pain in the right hip accompanied by tightness of the right leg. His right leg could straighten with great difficulty in walking and he was unable to walk or stand without support. He had noticed some difficulty with brushing teeth, tightening of the right arm and more recent slurring of speech with effortfulness and normal comprehension. There was no history of cranial nerve symptoms, bladder and sensory symptoms.
On examination, his blood pressure was 130/80 mmHg in the right upper limb. His pulse rate was 88 beats/minute respiratory rates were 18 breaths/minute and arterial oxygen saturation on room air was 98%. The central nervous system examination revealed facial hypomimia, fine, gaze evoked multi-directional nystagmus. He had spasticity of both upper and lower limbs (lower limb>upper limb) (right>left). His sensory system examination revealed no abnormality and deep tendon reflexes were normal. He had incoordination in both upper limbs, grossly abnormal finger nose testing, with terminal intentional tremors and dysmetria. He could stand with support and the gait was spastic and ataxic.
Investigations
His routine blood investigations revealed the following (Table 1).
| Investigations | Results | Normal values |
|---|---|---|
| Haemoglobin (g/L) | 13.3 | 14-17 |
| Total count (×109/L) | 7300 | 4.5-11.0 |
| Differential count (%) | NE: 70, LY: 20, MO: 8, EO: 2, BA: 0 | |
| Platelet count (×109/L) | 309000 | 150-350 |
| HIV, HBV, HCV serology | Negative | |
| Thyroid Stimulating Hormone(TSH) (mIU/L) | 1.592 | 0.4-4.2 |
| Serum sodium (mmol/L) | 140 | 135-145 |
| Serum potassium (mmol/L) | 3.6 | 3.5-5 |
| Serum creatinine (µmol/L) | 68.97 | 38-106 |
| Total and direct bilirubin (µmol/L) | 0.56/0.24 | 5-21/1.7-5.1 |
| Serum total protein/albumin (g/L) | 68/40 | 60-80/35-50 |
| Serum aspartate aminotransferase (U/L) | 24 | 10-35 |
| Serum alanine aminotransferase (U/L) | 30 | 10-40 |
| Serum alkaline phosphatase (U/L) | 87 | 30-120 |
| Prothrombin time | 18.6 | 11.7-16.1 |
| INR | 1.35 | |
| APTT | 34.9 | 27.8-40.4 |
| Calcium (mg%) | 10.45 | 8.3-10.4 |
| Phosphorous (mg%) | 3 | 2.5-4.6 |
| ANTI-PR3 and ANTI-MPO RU/mL | 5/<2 | <20,<20 |
| Vitamin B12 and Folic acid pgm/ml/ngm/ml | 541/10.3 | 200-950 |
| Copper ug% | 130 | 70-170 |
| CPK (CK) u/L | 105 | 45-195 |
| Vitamin D (25 OH) ng/ml | 11.9 | >30 |
| Glutamic acid decarboxylase autoantibody U/ml | >2000 | Negative <5.0;Positive>5.0 |
| CSF glucose [GRBS=88 mg/dl] CSF protein | CSF Glucose 59 CSF, protein 45 | |
| Cell counts CSF | T.WBC 2/CUMM (Normal) | |
| PCR for multiple viruses CSF, HSV,CMV,EBV,VZV and Adenovirus PCR | Negative | |
| ESR | 28 | |
| HbA1c (Glycosylated Hb) | 9.7 | <5.7 |
| ANTI Tissue Transglutaminase Antibody-ANTI-TTG | Negative | |
| ANTI-neuronal/onconeural antibody profile | GAD65-Positive ++ |
Table 1: Lab investigations.
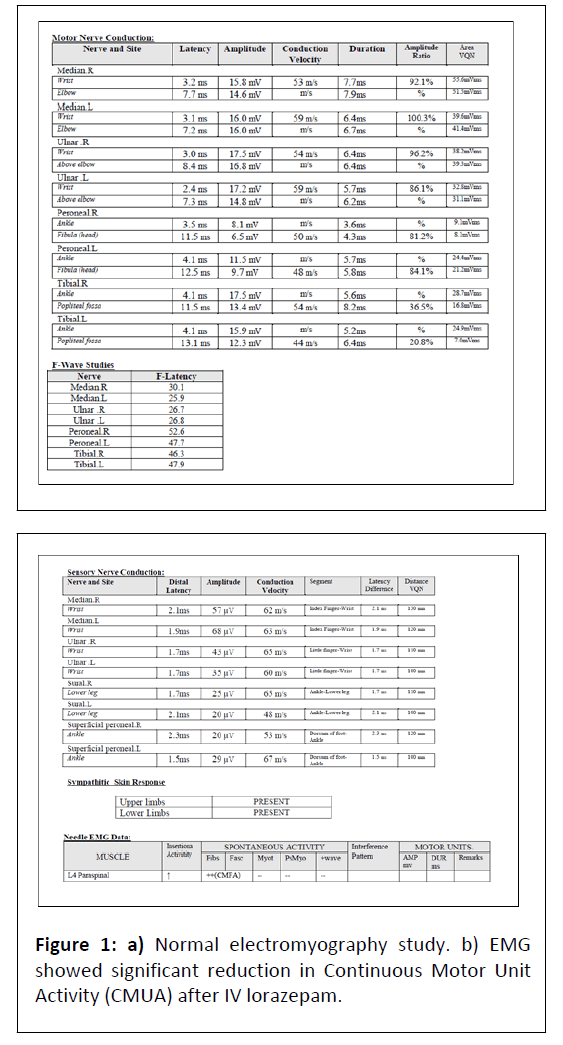
Both serum and CSF GAD antibody titres were elevated. Routine Nerve Conduction Velocity Studies (NCV) and Electromyography (EMG) were normal (Figure 1) paraspinal EMG showed Continuous Motor Unit Activity (CMUA) suppression with diazepam confirming the central origin of stiffness. Exteroceptive impulse testing neuro-physiologically revealed involvement of other muscle groups on stimulation of the median nerve suggestive of central hyper excitability. An MRI of the brain did not reveal any vascular lesions. MRI of the cervical spine was normal. A whole body FDG PET scan was done to look for paraneoplastic aetiology and was negative.
Differential diagnosis
This gentleman presented with a recent onset progressive asymmetric spastic ataxic syndrome, clinically localizing to the cerebellum/tracts along with corticospinal tracts. The aetiology considered were inflammatory [white matter disease/ autoimmune-disease/stiff-person syndrome/paraneoplastic orig -in] versus infection of central nervous system.
Treatment
In view of clinical presentation we suspected the stiff person spectrum with both serum and CSF GAD antibody titres being positive he was diagnosed as stiff person syndrome. He satisfied the criteria stiff person syndrome. He was started on symptomatic management with central sympatholytic agents and diazepam.
A total 5 cycles of plasmapheresis and 1 gm methylprednisolone for 5 days was given. Home based physiotherapy was advised. Rituximab 600 mg was given as second line immunosuppressive agent.
Outcome and follow-up
Injection rituximab 600 mg once a week for total four weeks was planned. Injection methylprednisolone for 500 mg once a week for six weeks and 250 mg once a week for four weeks was planned.
At the end of six weeks there was significant improvement in spasticity and ataxia and he could walk without support.
Results and Discussion
Stiff-person syndrome is an uncommon disorder characterized by progressive muscle stiffness, rigidity and spasm involving the axial muscles, which results in severely impaired ambulation [1].
Based on several observations, the role of an autoimmune component in the pathogenesis of stiff-person syndrome including an association with type 1 diabetes mellitus and other autoimmune disorders has been suggested. Glutamic acid decarboxylase is the rate-limiting enzyme for Gamma Amino Butyric Acid (GABA) synthesis.
As GABA is the major inhibitory neurotransmitter in the central nervous system, the dysfunction of GABAergic pathways due to presence of autoantibodies is believed to be involved in the pathogenesis of SPS [2-4]. There is an association between anti-Glutamic Acid Decarboxylase (GAD) antibodies and stiff person syndrome. These antibodies target GABAergic neurons and their nerve terminals [5,6]. In patients positive for anti-GAD antibodies, there is a strong association with other autoimmune diseases, like insulin-dependent DM, hypothyroidism, grave’s disease and pernicious anemia. It is currently believed that one-third to two-thirds of patients with SPS are accompanied by DM [7].
There are three subtypes of stiff person syndrome.
• Classic SPS, being the most common where patients present with truncal stiffness, generalized rigidity and frequent muscle spasms.
• Partial SPS, in which there is involvement of one limb, or a localized group of muscle.
• Paraneoplastic SPS variant, which is extreme rare form and these patients, are usually Glutamic Acid Decarboxylase (GAD) antibody-negative. Most common malignancies include breast and lung cancer and Hodgkin lymphoma [8].
To diagnose a patient with stiff person syndrome requires a high index of suspicion. Diagnosis of stiff person syndrome is generally based on following criteria’s [8,9].
• Stiffness in the axial and limb muscles causing ambulatory impairment.
• Presence of episodic spasms which are precipitated by sudden movement, noise, or emotional upset.
• A positive therapeutic response to oral diazepam or findings of continuous motor-unit activity on Electromyography (EMG) which are abolished by intravenous diazepam.
• Absence of other neurologic disorders explaining the clinical scenario.
Investigations for SPS include basic laboratory studies including CBC, testing for anti-GAD antibody in blood and CSF, electromyography, imaging of neuro-axis to rule out degenerative, infectious, malignant, or inflammatory diseases.
Treatment strategies for SPS are broadly divided into two categories: The first category includes GABA-enhancing drugs and the second category includes immunomodulatory agents like glucocorticoids, IVIG, anti-CD20 (rituximab), plasma exchange [10-13].
We treated our patient using both categories of agents and ultimately our patients showed favorable outcome.
Conclusion
SPS is a rare disorder and high index of suspicion is required for diagnosing a case with SPS. Presence of Anti-GAD antibody provides an important clue for diagnosing SPS.
In patients diagnosed with SPS screening for other autoimmune diseases such as hypothyroidism, Grave’s disease and pernicious anaemia in addition to insulin dependent DM should be considered. Early diagnosis and appropriate treatment improves prognosis.
References
- Helfgott SM (1999) Stiff-man syndrome: From the bedside to the bench. Arthritis Rheum 42: 1312-20.
[Crossref], [Google Scholar], [Indexed]
- Dalakas MC (2009) Stiff-person syndrome: Advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol 11: 102-10.
[Crossref], [Google Scholar], [Indexed]
- Gershanik OS (2009) Stiff-person syndrome. Parkinsonism Relat Disord 15: S130-134.
- Holmøy T, Geis C (2011) The immunological basis for treatment of stiff person syndrome. J Neuroimmunol 231: 55-60.
[Crossref], [Google Scholar], [Indexed]
- Solimena M, Folli F, Denis-Donini S, Comi GC, Pozza G, et al. (1988) Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy and type I diabetes mellitus. N Engl J Med 318: 1012-20.
[Crossref], [Google Scholar], [Indexed]
- Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P (1990) Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med 322: 1555-60.
[Crossref], [Google Scholar], [Indexed]
- Blum P, Jankovic J (1991) Stiff-person syndrome: An autoimmune disease. Mov Disord 6: 12-20.
[Crossref], [Google Scholar], [Indexed]
- Baizabal-Carvallo JF, Jankovic J (2015) Stiff-person syndrome: Insights into a complex autoimmune disorder. J Neurol Neurosurg Psychiatry 86: 840–8.
[Crossref], [Google Scholar], [Indexed]
- Lorish TR, Thorsteinsson G, Howard FM (1989) Stiff-man syndrome updated. Mayo Clin Proc 64: 629-36.
[Crossref], [Google Scholar], [Indexed]
- Barker RA, Marsden CD (1997) Successful treatment of stiff man syndrome with intravenous immunoglobulin. J Neurol Neurosurg Psychiatry 62: 426-7.
[Crossref], [Google Scholar], [Indexed]
- Pagano MB, Murinson BB, Tobian AAR, King KE (2014) Efficacy of therapeutic plasma exchange for treatment of stiff-person syndrome. Transfusion 54: 1851-1856.
[Crossref], [Google Scholar], [Indexed]
- Baker MR, Das M, Isaacs J, Fawcett PRW, Bates D (2005) Treatment of stiff-person syndrome with rituximab. J Neurol Neurosurg Psychiatry 76: 999-1001.
[Crossref], [Google Scholar], [Indexed]
- Dupond JL, Essalmi L, Gil H, Meaux-Ruault N, Hafsaoui C (2010) Rituximab treatment of stiff-person syndrome in a patient with thymoma, diabetes mellitus and autoimmune thyroiditis. J Clin Neurosci 17: 389-91.
[Crossref], [Google Scholar], [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences