Sleep Disordered Breathing in the Lung Transplant Population, a Case Report and Literature Review
Brian Palen, Tiffany M Bridges, Vishesh K Kapur and Elizabeth C Parsons
DOI10.21767/2471-8041.100029
Brian Palen*, Tiffany M Bridges, Vishesh K Kapur and Elizabeth C Parsons
Puget Sound Veterans Hospital and Clinic, University of Washington, USA
- *Corresponding Author:
- Brian Palen
Puget Sound Veterans Hospital and Clinic, University of Washington, USA.
Tel: +1 206-543-2100
E-mail: Brian.palen@va.gov
Received date: April 08, 2016; Accepted date: June 30, 2016; Published date: July 07, 2016
Citation: Palen B, Bridges TM, Kapur VK, et al. Sleep Disordered Breathing in the Lung Transplant Population, a Case Report and Literature Review. Med Case Rep. 2016, 2:3. doi: 10.21767/2471-8041.100029
Abstract
Background: Patients waiting for and having received lung transplantation commonly demonstrate known risk factors for sleep disordered breathing (SDB) including central sleep apnea (CSA). However, little is known is about the prevalence or clinical implications of SDB in this patient population.
Methods: We report a 61 year old man who underwent uncomplicated bilateral lung transplantation. Approximately 5 weeks post-transplant, the patient developed fatigue, somnolence, and mild intermittent confusion. Extensive cardiologic and neurologic evaluations were unrevealing regarding cause of symptoms. Diagnostic polysomnogram revealed severe central sleep apnea with a periodic respiratory pattern.
Results: The patient was initiated on positive airway pressure therapy during sleep with an adaptive servoventilation device which successfully treated central sleep apnea and improved daytime symptoms. A repeat diagnostic polysomnogram performed 8 months post-transplant showed resolution of central sleep apnea.
Conclusion: SDB including CSA is a poorly understood occurrence in the lung transplantation patient population that warrants clinical attention and further investigation.
Case Report
A 61 year old man underwent bilateral lung transplantation for respiratory failure due to severe chronic obstructive pulmonary disease (COPD).The surgical course was without significant complication and the patient was extubated without difficulty. The immediate post-operative period was significant for an isolated episode of atrial fibrillation with rapid ventricular response requiring cardioversion. Standard immunosuppression and rehabilitation were initiated per protocol, and the patient was discharged to home approximately 3 weeks post-transplant.
5 weeks post-transplant, the patient noted progressive fatigue and daytime sleepiness as well as intermittent mild confusion and a single near-syncopal event. The patient denied anginal chest discomfort, signs of heart failure, or focal neurologic findings. On further questioning, the patient reported frequent nocturnal awakenings, non-restorative sleep, daytime somnolence and his care-taker noted the patient to have respiratory pauses in his sleep which occurred in a cyclical pattern.
On physical examination the patient was afebrile, normotensive, had a room-air saturation of 97%, was neurologically intact, and demonstrated no findings of heart failure. Serologic laboratory evaluation was without evidence of cardiac ischemia, renal or other organ failure. Electrocardiogram and telemetry demonstrated sinus rhythm without ischemia, ectopy or arrhythmia. Transthoracic echocardiogram revealed normal bilateral ventricular size and function with estimated pulmonary arterial systolic pressure of 30 mmHg. CT scan of the chest showed normal post-surgical changes with no evidence of pulmonary emboli or parenchymal lung abnormalities. Neurologic evaluation including EEG as well as brain CT and MRI were without significant finding.
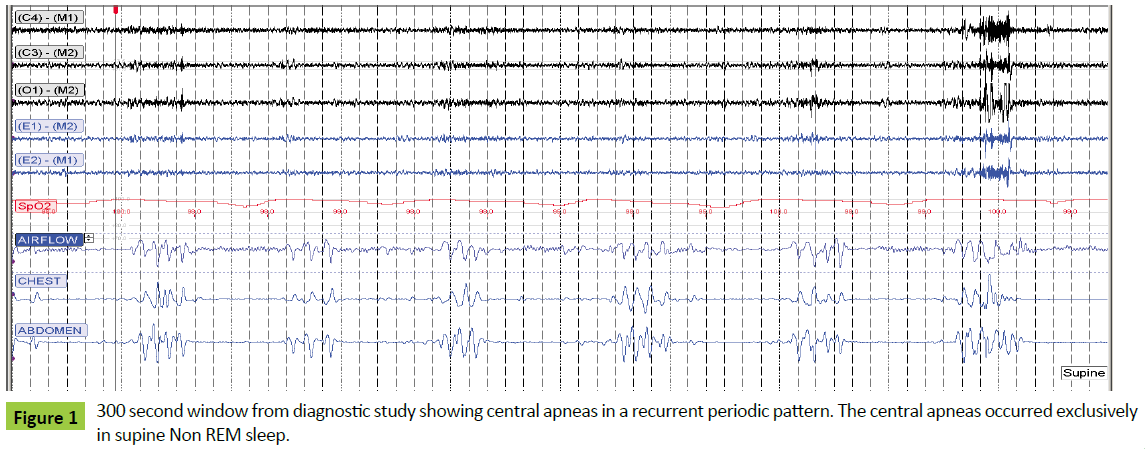
A laboratory based diagnostic polysomnogram (PSG) was performed approximately six weeks post-transplant, and showed an apnea hypopnea index (AHI) of 56 consisting of predominantly central apnea events. The central apneas occurred exclusively in supine non-REM sleep and were characterized by a periodic occurrence with a respiratory pattern which was at times crescendo-decrescendo in waveform and had a cycle time of 35-40 seconds (Figure 1). The study was performed on room air and 100% of the patient’s sleep time was spent with oxygen saturations greater than 90%.
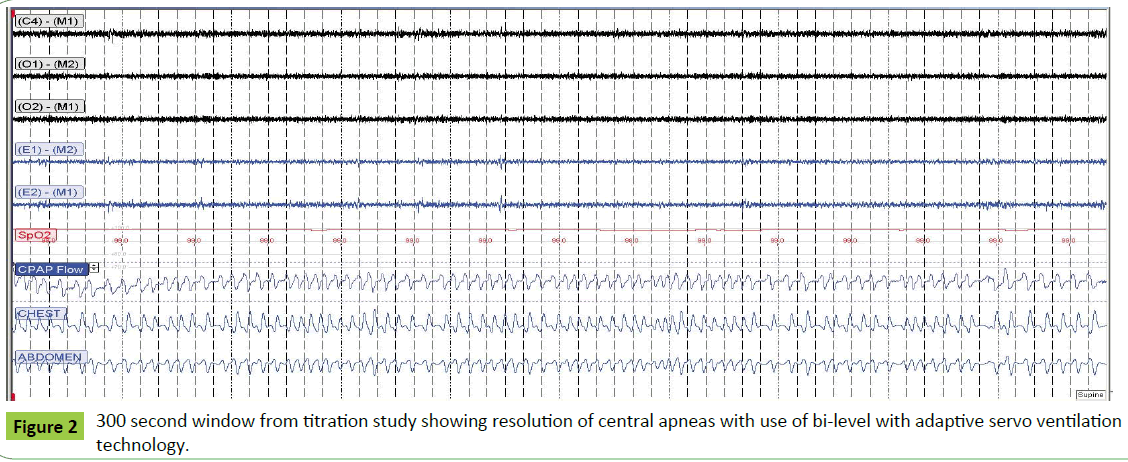
A laboratory based titration PSG was subsequently performed with persistence of central apnea events and periodic breathing with continuous positive airway pressure (CPAP) titration. Bi-level with adaptive servoventilation technology was then initiated, with an immediate resolution of central events (Figure 2). The patient was subsequently initiated on adaptive servo ventilation therapy, and noted a significant improvement in his fatigue, somnolence and confusion.
8 months post-transplant, a repeat laboratory based diagnostic PSG revealed no evidence of central sleep apnea or periodic breathing and only mild obstructive SDB with AHI of 7 and no significant hypoxia. The patient was subsequently switched to standard CPAP therapy with good clinical response.
Discussion
Lung transplantation is a validated treatment for advanced COPD. Commonly recognized post-transplant occurrences include acute rejection, infection, bronchiolitis obliterans syndrome (BOS), gastro esophageal reflux disease (GERD), hypertension, and depression [1,2]. Less understood is the prevalence, clinical presentation of, risk factors for, and optimal treatment strategies of SDB in this population. SDB encompasses a group of diagnoses including CSA, obstructive sleep apnea (OSA), sleep related hypoventilation, and sleep related hypoxemia [3]. Risk factors for SDB in the general population include obesity, large neck circumference, male gender, age, cardiologic disorders, and underlying lung disease [4,5]. Additional incurred risk factors for SDB in lung transplantation patients include acute weight changes, thoracic volume and pressure changes, altered chemoresponsiveness, lung denervation, and use of narcotic as well as mineralocorticoid altering medications.
CSA is defined by the cessation of airflow without concurrent respiratory effort, and may clinically present in a broad range of respiratory patterns [6]. This diversity is reflective of the complex physiologic regulation, which includes contributions from chemo-receptor responsiveness, circulatory response times, and ventilatory capacity. An established mode of classification for central apnea is based on the concept of loop gain, defined as the response to a disturbance (ventilatory response) in ratio to the disturbance itself (apnea). Components of the overall loop gain of the system include; controller gain (chemo-responsiveness), plant gain (ventilatory efficiency), and mixing gain (including circulatory times and gas delivery) [7]. High loop gain models such as Cheyne-Stokes and other periodic respiratory patterns have a ratio of ≥ 1, resulting in an easily destabilized ventilatory system.
High loop gain CSA models are common in patients with congestive heart failure, atrial fibrillation, cerebral vascular accident, and renal failure [8], a specific cause for the occurrence of central apnea in our patient is not clearly established. Our patient had no history of SDB prior to lung transplantation, and at the time of presentation demonstrated no clinical evidence of a neurologic or cardiogenic process. Additionally, while daytime fatigue and sleepiness can be associated with central apnea, an association with our patient’s confusion and near-syncopal episode is less supported. That said, it is feasible that in the setting of recent transplantation, our patient developed a high-gain system due to a large increase in plant gain (transplanted lungs) with additional increases in controller and mixing gain which subsequently equilibrated over time.
Although it is not unexpected that patients with respiratory failure awaiting lung transplantation would have a high prevalence of SDB, there is surprisingly little data from quality studies to guide medical decision-making. In a prospective study, Malouf et al. performed room air PSG on 80 patients awaiting lung transplantation. 32 of 80 (40%) patients met SDB criteria defined as a respiratory disturbance index (RDI) ≥ 10 (central and obstructive events were not distinguished), or ≥ 10% of sleep time with oxygen saturations ≤ 90% [9]. A retrospective review of 60 patients with end-stage lung disease undergoing evaluation for lung transplantation demonstrated 40 of 60 (67%) to have OSA (defined by RDI ≥ 5, with no data on central apnea events provided) with the majority having moderate-to-severe OSA. While there was no association between underlying diagnosis and the presence of OSA, associations were found between AHI severity and higher DLCO% as well as lower nocturnal oxygen saturations [10].
There is a similar paucity of data regarding the occurrence of SDB after lung transplantation. In the previously mentioned study, Malouf et al. completed post-transplant PSG on 25 patients including 11 who had met SDB criteria prior to transplantation. SDB resolved in 6 of the 11 (55%) patients with prior SDB, but new SDB developed in 4 of 14 (29%) patients without prior SDB findings. The presence of SDB did not impact pre or posttransplant survival. A single center cross sectional study of 24 patients at least one year post-transplant showed 15 of 24 (63%) to have SDB (defined as AHI ≥ 10), with 25% demonstrating predominant CSA. Patients who developed SDB were shown to have elevated systolic blood pressure, greater age, greater change in BMI, more recent date of transplantation, diagnosis of emphysema, observed snoring, and an association between Cyclosporine use and central apnea was suggested (P=0.006) [11]. Our patient was not taking Cyclosporine, and further, its use has not been associated with central apnea in other solidorgan transplant populations [12,13]. Lastly, a prospective study evaluating risk factors for BOS performed PSG in 14 posttransplant patients and found 100% to meet SDB criteria (defined as AHI>5) with 3 patients demonstrating mild central apnea activity. No associations between SDB and esophageal reflux or SDB and BOS were found.
Conclusion
Our case report and literature review highlight the uncertainties to the frequency as well as spectrum of clinical presentations by which SDB and in particular CSA may present in patients prior to and after lung transplantation. Despite growing data showing that SDB is common in this patient population, there are no established screening guidelines or treatment recommendations. Thus, data from additional longitudinal studies are needed to identify patient risk factors as well as to clarify the impact of SDB on unique clinical consequences in these patients such as health related quality of life, depression, GERD, hypertension and bronchiolitis obliterans syndrome. In the interim, providers treating this patient population should have a low threshold to screen for SDB and should treat accordingly.
References
- Shepherd K (2008) Obstructive sleep apnoea and nocturnal gastroesophageal reflux are common in lung transplant patients. Respirology 13 :1045-1052.
- Ahmad S, Shlobin O, Nathan S (2011) Pulmonary complications of lung transplantation. Chest 139: 402-411.
- Darien I (2014) International Classification of Sleep Disorders. (3rd edn), American Academy of Sleep Medicine, United States.
- Tufik S (2010) Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 11: 441-446.
- Young T, Skatrud J, Peppard P (2004) Risk factors for obstructive sleep apnea in adults. JAMA 291: 2013-2016.
- Javaheri S (2010) Central sleep apnea. Clin Chest Med 31:235- 248.
- Naughton M (2010) Loop gain in apnea: gaining control or controlling the gain?Am J RespirCrit Care Med 181:103-105.
- Javaheri S (1999) A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 341: 949-954.
- Malouf M (2008) Sleep-disordered breathing before and after lung transplantation. J Heart Lung Transplant 27:540- 546.
- Romem, A (2013) Obstructive sleep apnea in patients with end-stage lung disease. J Clin Sleep Med9:687-693.
- Naraine V, Bradley T, Singer L (2009) Prevalence of sleep disordered breathing in lung transplant recipients. J Clin Sleep Med 5: 441-447.
- Molnar M (2007) High prevalence of patients with a high risk for obstructive sleep apnoea syndrome after kidney transplantation--association with declining renal function. Nephrol Dial Transplant 22: 2686-2692.
- Brilakis E (2000) Sleep apnea in heart transplant recipients: type, symptoms, risk factors and response to nasal continuous positive airway pressure. J Heart Lung Transplant 19:330-336.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences