Primary Autoimmune Neutropenia in Infancy Presenting with Agranulocytosis: A Case Report
Tharindi Suriapperuma1* , Zainab Razeen2, Chinthaka Botheju2and Manel Panapitiya2
1Department of Pediatrics, University of Kelaniya, Ragama, Sri Lanka
2Department of Pediatrics, North Colombo Teaching College, Ragama, Sri Lanka
- *Corresponding Author:
- Tharindi Suriapperuma, Department of Pediatrics, University of Kelaniya, Ragama, Sri Lanka, Tel: 94715121047; E-mail: thari1707@gmail.com
Received date: May 02, 2022, Manuscript No. IPMCRS-22-12691; Editor assigned date: May 05, 2022, Pre QC No. IPMCRS-22-12691 (PQ); Reviewed date: May 20, 2022, QC No. IPMCRS-22-12691; Revised date: July 04, 2022, Manuscript No. IPMCRS-22-12691 (R); Published date: July 12, 2022, DOI: 10.36648/2471-80 41.08.07.234
Citation: Suriapperuma T, Razeen Z, Botheju C, Panapitiya M (2022) Primary Autoimmune Neutropenia in Infancy Presenting with Agranulocytosis: A Case Report. Med Case Rep Vol:8 No:7
Abstract
Primary autoimmune neutropenia in infancy is a rare, acquired condition with an incidence of 1 in 100,000. It has good prognosis with recovery almost always around the age of two years. Here, we report an infant presented with agranulocytosis secondary to primary autoimmune neutropenia.
A 9-month-old girl, born to healthy non-consanguineous parents presented with a mild acute illness and found to have agranulocytosis. She was observed with serial full blood counts and cyclical neutropenia was excluded. Other cell lines in the full blood count and immunoglobulin levels were normal. Bone marrow aspiration revealed normocellular granulopoiesis with mild impairment of mature neutrophils. There was no evidence of hematological malignancy or myelodysplasia. At the age of one year and four months, she presented with acute bacterial infection and there was a 20 times rise in absolute neutrophil count. During the latter part of the illness, she redeveloped severe neutropenia and was treated with granulocytic colony stimulation factor.
This case report highlights the key differentiating features of autoimmune neutropenia from congenital neutropenia and the importance of being aware of this rare condition to prevent unnecessary interventions despite frightening neutropenia.
Keywords
Primary autoimmune neutropaenia in infancy; Agranulocytosis; Granulocytic colony stimulation factor; Congenital neutropenia’s
Introduction
Absolute Neutrophil Count (ANC) less than 1500/µL is defined as neutropenia [1]. If neutropenia persisting more than three months it is considered as chronic neutropenia [2]. Primary Autoimmune Neutropenia (AIN) in infancy is a rare, acquired condition with an incidence of 1 in 100,000 but relatively common than Congenital Neutropenias (CN) (3). Here, we report an infant presented with agranulocytosis (ANC<200 µL) secondary to primary autoimmune neutropenia.
Case Presentation
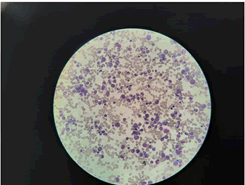
A 9-month-old girl, who was apparently well, born to healthy non-consanguineous parents presented with loose stools for 1-week duration. Her ANC was 180/µL at the time of admission. Neutropenia was persisted throughout. She was observed with serial full blood counts and cyclical neutropenia was excluded. Other cell lines in the full blood count and immunoglobulin levels were normal. Bone Marrow (BM) aspiration revealed normocellular granulopoiesis with mild impairment of mature neutrophils. There was no evidence of haematological malignancy or myelodysplasia (Figure 1). During follow up she did not develop severe or recurrent infections despite persistent moderate and severe neutropenia. So, she was not offered long term medications. Viral etiologies such as Cytomegalovirus, Epstein-Barr virus, Parvo B19, and Human immune deficiency virus were excluded. Her mycoplasma serology was negative.
At the age of one year and four months, she presented with high grade fever and that episode was treated as sepsis. Her ANC was 6800/µL with total white cell count of 13680/µL during the first 24 hours of the acute illness. One week prior to this acute illness her ANC was 340/µL. There was a marked rise in neutrophil number with this bacterial infection. During the latter part of the illness, she redeveloped severe neutropenia and she was commenced on Granulocytic Colony Stimulation Factor (G-CSF) 2 µg/kg for three days and ANC raised up to 3500/µL.
She was developmentally age appropriate and thriving well. She was vaccinated age appropriately. She was not on long term medications in the past. She has no dysmorphism, lymphadenopathy, or hepatosplenomegaly. The rest of the system examination was unremarkable.
Discussion
AIN in childhood is the commonest chronic neutropenia in children [1]. The estimated prevalence is 1 in 100,000 children under ten years of age. The neutrophils are destroyed by autoantibodies against neutrophil‑specific cell surface antigens, such as the human neutrophil antigen HNA‑1a, HNA-1b and HNA-2. Antibody coated neutrophils are destructed in the peripheral blood [3,4].
Many of them are asymptomatic. Unlike individuals with CN, individuals with AIN have adequate bone marrow storage of neutrophils. However, they have a very low neutrophil count in the peripheral circulation because of peripheral destruction of neutrophils by anti-neutrophil antibodies. However demonstration of anti-neutrophil antibodies has little or no role in establishing the diagnosis of AIN as most tests have significant false positive or false negative rates [1].
There are well recognized salient features of AIN in infancy. They usually present between 8 to 11 months of age. These individuals get few or minor infections. Severe or invasive infections are rarely seen despite very low neutrophil count. In AIN BM has normal or increased myelopoiesis and may show a decrease in mature neutrophils [1-5]. The ANC rises with bacterial infections or stress which rules out hypo-productive BM. It is suggestive of an adequate stored pool of neutrophils in the BM. Hypo-productive BM is seen in CN. A study done by Bux J et al. described that 97% of patients with AIN in infancy had normal or increased cellularity with or without a reduced number of metamyelocytes, bands, and mature neutrophils in the BM examination [6].
Our patient demonstrated all the features that are mentioned above. We have excluded humoral immunodeficiencies by demonstrating normal immunoglobulin’s. Systemic autoimmune disorders giving rise to AIN is rare in younger age and our patient did not show any clinical features as well. On that ground, we diagnosed primary AIN in infancy in our patient.
The Glucocorticoid Stimulation Test (GST) can be done to rule out hypo-productive BM by demonstrating a twofold or greater rise in ANC after administrating a single dose of prednisolone 1-2 mg/kg, if adequate ANC was not identified during an acute bacterial infection [1]. As our patient had a 20 times rise is ANC with bacterial infection we did not perform the GST.
Children with AIN usually do not require specific treatment for neutropenia or antibiotic prophylaxis as they do not frequently suffer from infections [6]. Acute infections need to be treated promptly. G-CSF has a place in the event of serious infection with neutropenia. The recommended dose is 1-2 µg/kg for nearly five days and the goal is to keep ANC above 1000/µL during an infection [3]. Unlike CN, acquired neutropenia do not have the risk of development of myelodysplasia or leukaemia following G-CSF therapy. There is a vigorous response to G-CSF is observed in children with AIN causing severe bone pain [1].
Neutropenia diet, use of antibacterial local applications, and household disinfectants are not recommended for children with AIN. Good dental hygiene is encouraged and should follow routine immunization.
The prognosis is good in AIN in childhood and recovery is observed in almost all patients in the 2nd or 3rd year of life [7]. Comparative to AIN in childhood, children with CN present quite early in life with severe recurrent infections and have hypo-productive BM. They do not recover as AIN in childhood [8].
Conclusion
AIN in childhood is a rare, acquired cause of severe neutropenia with less frequent, mild infections and recovery almost always around the age of two years. This case report highlights the key differentiating features of AIN from CN and the importance of being aware of this rare condition to prevent unnecessary interventions despite frightening neutropenia.
References
- Newburger PE (2016) Autoimmune and other acquired neutropaenias. Hematology Am Soc Hematol Educ Program 2:38–42
[Crossref] [Google Scholar] [Pubmed]
- Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB (2011) Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis 6:26
[Crossref] [Google Scholar] [Pubmed]
- Jinca C, Serban M, Ursu E, Munteanu A, Arghirescu S (2021) Primary autoimmune neutropenia of infancy and childhood in a cohort of patients from western Romania. Exp Ther Med 21:280
[Crossref] [Google Scholar] [Pubmed]
- Farruggia P, Dufour C (2015) Diagnosis and management of primary autoimmune neutropaenia in children: insights for clinicians. Ther Adv Hematol 6:15-24
[Crossref] [Google Scholar] [Pubmed]
- Celkan T and Koc BS (2015) Approach to the patient with neutropenia in childhood. Turk Pediatri Ars 50:136–144
[Crossref] [Google Scholar] [Pubmed]
- Bux J, Behrens G, Jaeger G, Welte K (1998) Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood 91:181-186
[Crossref] [Google Scholar] [Pubmed]
- Capsoni F, Sarzi-Puttini P, Zanella A (2005) Primary and secondary autoimmune neutropenia. Arthritis Res Ther 7:208–214
[Crossref] [Google Scholar] [Pubmed]
- Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K (2017) Severe congenital neutropenias. Nat Rev Dis Primers 8:17032
[Crossref] [Google Scholar] [Pubmed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences