Posterior Neuroblastoma with Spinal Cord Extension Presenting at an Unusual Age: Case Report with Literature Review
Jihad Boularab*, Hind Sahli, Chaimae Lahlou, Siham El Haddad, Nazik Allali and Latifa Chat
Department of Radiology, Children Hospital of Rabat (HER), Mohammed V University, Rabat, Morocco
- *Corresponding Author:
- Jihad Boularab
Department of Radiology,
Children hospital of Rabat (HER), Mohammed V University, Rabat,
Morocco,
E-mail: boularabJihad@gmail.com
Received date: August 16, 2022, Manuscript No. IPMCRS-22-14380; Editor Assigned date: August 19, 2022, PreQC No. IPMCRS-22-14380 (PQ); Reviewed date: August 29, 2022, QC No. IPMCRS-22-14380; Revised date: September 08, 2022, Manuscript No. IPMCRS-22-14380 (R); Published date: September 15, 2022, DOI: 10.36648/2471-8041.8.8.240.
Citation: Boularab J, Sahli H, Lahlou C, Haddad SE, Allali N, et al. (2022) Posterior Neuroblastoma with Spinal Cord Extension Presenting at an Unusual Age: Case Report with Literature Review. Med Case Rep Vol.8 No.8: 240.
Abstract
Neuroblastoma is the most common extracranial malignancy in children. The abdomen, more specifically the retroperitoneum, is the site of predilection, followed by the mediastinum, which is less common. We present the case of a 10-years-old boy presenting a mediastinal neuroblastoma with intraspinal extension and paraparesis, as well as respiratory symptoms.
Keywords
Neuroblastoma spinal extension children; Childhood tumor; Ganglioneuroma
Introduction
Neuroblastoma is the third most common childhood tumor. Imaging is crucial in the diagnosis, staging, treatment planning, and response evaluation and follow-up. CT is currently the modality of choice due to its accessibility and ability to detect calcification within the tumor as well as establishing local extension, lymph node involvement and non-skeletal metastases.
Case Report
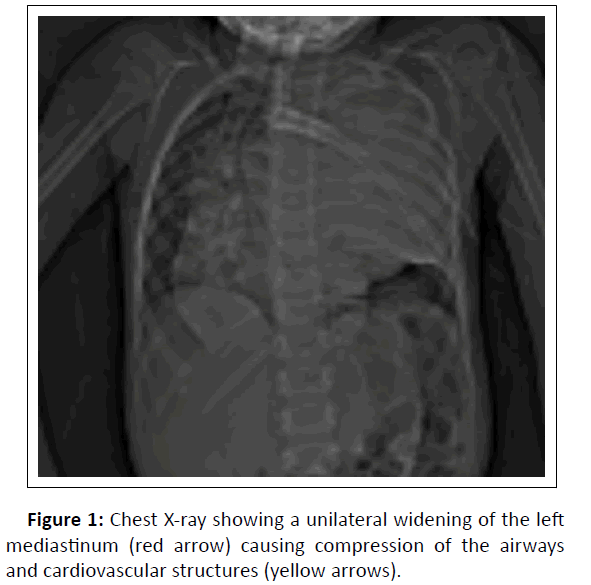
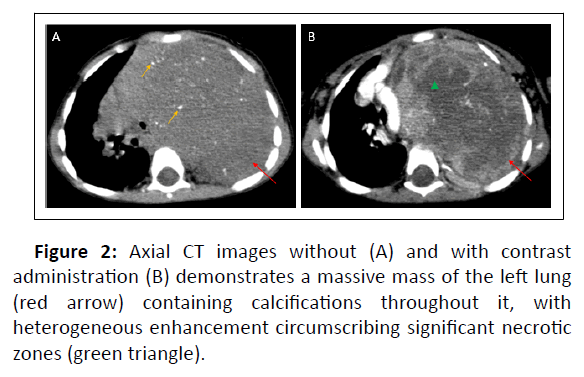
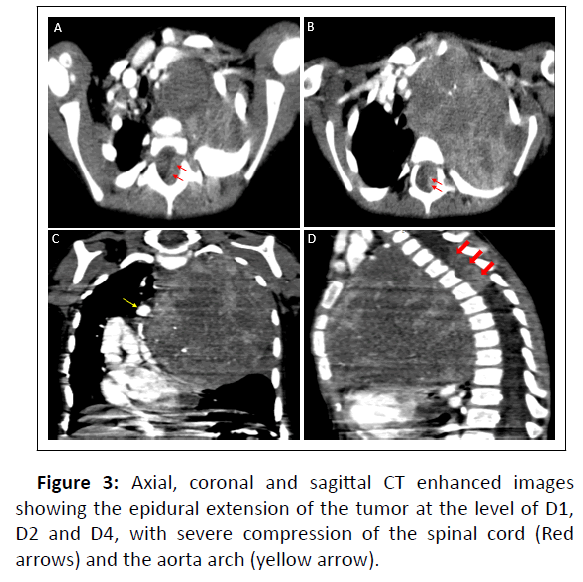
We report a case of a 7years old boy; chest X-ray showed heterogeneous opacity with indistinct borders involving most of the left lung causing compression of the airways and cardiovascular structures (Figure1). We completed by a thoracic CT scan which revealed a large, well-circumscribed mass in the upper and posterior mediastinum, occupying nearly the entire left pulmonary hemifield, with heterogeneous density containing calcifications and areas of necrosis interposed between areas of enhancing tumor tissue (Figure 2). The CT also demonstrated the extension of the tumor to the spinal cord at multiple levels with compression and distortion of the calsac (Figure 3).
Discussion
Neuroblastoma, along with ganglioneuroma and ganglioneuroblastoma, constitute a group of tumors that originates from the lymph nodes. It often occurs in infants during the first 10 years mostly before the age of 2, with a male predominance [1].
The abdomen, specifically the retroperitoneum, is the most common primary site, with the adrenal gland accounting for two-thirds of all lesions; however, it can also develop in the Zuckerkandl organ or follow the sympathetic ganglia distribution along the paraspinal areas from the neck to the pelvis. The second location of preference is the mediastinum, which is said to extend to the spinal cord in 10%-15% of cases. The pelvis and the neck are two less common sites [2].
Bone, bone narrow, liver, lymph nodes and skin are the most common sites of metastasis, with at least 70% of patients having disseminated illness at the time of diagnosis. Rarely, even with a metastatic disease context, there is no detectable primary tumor [2].
On the clinical side, children around the age of 2 usually present with respiratory symptoms, but the tumor may manifest as a result of neural compression. In this case symptoms vary depending on the extent of this compression ranging from pain and motor or sensory deficit to sphincter dysfunction. Furthermore, 50% of the patients experience long-term severe consequences [3].
Imaging
Chest radiography
Plain radiographs still quite helpful, almost always showing a well-defined tumor located in the paravertebral region within the posterior mediastinum, containing calcifications in 25% of the cases, with a possible mass effect on the adjacent structures [1].
Rib erosion is less common than other malignancies such as Ewing sarcoma and PNET [4].
Specific features can be seen in posterior mediastinal lesions such as splaying of the adjacent posterior ribs, the enlargement of the paraspinal lines and the erosion of the surrounding vertebral pedicles suggesting intraspinal extension [3].
The examination of the extra thoracic areas may provide additional diagnostic evidence, such as metaphyseal lucency on the humeral head (resulting of metastatic infiltration) or other cortical bone abnormalities [4].
Additional skeletal radiographs are likely to be part of the initial imaging if the child reports bone pain and can show a discrete lytic regions or metaphysal lucencies which are typical patterns of metastatic lesions. However a normal bone appearance does not exclude the possibility of metastatic lesions [4].
Ultrasonography
In the paedratric population, ultrasound is a good screening method for detecting an abdominal or pelvic mass. It is frequently used as the first line of investigation. On US, NBLs appear as solid, heterogeneous masses with calcification, but they are rarely cystic [5]. It is also used to determine the local extent of the initial tumor (if intra-abdominal, intrapelvic) and to establish a baseline for follow-up in neuroblastoma [4].
Computed Tomography (CT)
CT is the first cross-sectional imaging study used to characterize a thoracic neuroblastoma, as it is widely available in imaging departments and today's multidetector CT devices allow extremely quick acquisitions with no motion artifacts, minimizing the need for sedation. Intraspinal extension is also adequately shown and multiplanar reconstructions for accurate coronal and sagittal views are possible.
NB is commonly observed on computed tomography as a massive, lobulated, heterogeneous solid mass that displaces and encases nearby organs and arteries rather than invading them [6]. In 85 percent of abdominal and 50 percent of thoracic NBLs, coarse, finely stippled, or curvilinear calcifications can be found. Low attenuation regions within the tumor suggest pseudonecrosis or hemorrhage [3]. The diagnosis of NB is suggested by conglomerate nodal masses with calcification or a paravertebral mass with intra spinal extension [6].
On another way, CT is the gold standard for determining locoregional extension and detecting metastatic disease [3]. Metastases are most common in the bone marrow (70 percent) or bone (55 percent), with the liver being less common appearing under two possible forms: Diffuse infiltration, which can be difficult to detect on CT, or focal nodular hypodensities, which are more common in older children with stage 4 disease.
Lung metastases are uncommon, occurring in just about 3% of cases [3].
MRI
Although both CT and MRI are excellent for demonstrating retrocrural and paravertebral extension, MRI is the preferred imaging modality in patients with intra spinal extension of primary paraspinal tumors due to its superior visualization of the spinal cord, nerve roots, and subarachnoid spaces [7].
When it comes to determining marrow infiltration and tumor intraspinal extension, MRI outperforms CT. The lack of ionising radiation and the absence of the need for oral contrast add to the benefits of MRI [3].
The tumor is usually heterogeneous, with varied enhancement, prolonged T1 and T2 relaxation durations, and low and high signal intensity on T1W and T2W images, respectively, which reveal little or no enhancement. The tumor's cystic and hemorrhagic regions can be consistently detected. NBLs show a stronger signal on diffusion-weighted imaging due to the restricted diffusion of water protons within the solid tumor matrix [3].
Moreover, MRI allows for a more accurate assessment of epidural NBL extension and leptomeningeal dissemination [3].
Bone marrow disease appears as diffuse infiltration on T1W and T2W imaging, although it can also appear as a nodular pattern with areas of low and high signal strength [3].
Conclusion
It is unusual for a primary posterior neuroblastoma to manifest with paraparesis. Imaging is critical in identifying tumor marrow infiltration and intraspinal extension. Finally, A multidisciplinary approach is required to improve the prognosis of this condition, and treatment options differ depending on risk stratification.
References
- Bousvaros A, Kirks DR, Grossman H (1986) Imaging of neuroblastoma: An overview. Pediatr Radiol 16: 89–106.
[Crossref], [Google Scholar], [Indexed]
- Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, et al. (2003) Neurogenic tumors in the abdomen: Tumor types and imaging characteristics. Radiographics 23: 29-43.
[Crossref], [Google Scholar], [Indexed
- Papaioannou G, Hug KM (2005) Neuroblastoma in childhood: Review and radiological findings. Cancer Imaging 5: 116–127.
[Crossref], [Google Scholar], [Indexed]
- Hiorns M, Owens C (2001) Radiology of neuroblastoma in children. Eur Radiol 11: 2071–2081.
[Crossref], [Google Scholar], [Indexed]
- Dumba M, Jawad N, McHugh K (2015) Neuroblastoma and nephroblastoma: A radiological review. Cancer Imaging 15: 5.
[Crossref], [Google Scholar], [Indexed]
- Kembhavi SA, Shah S, Rangarajan V, Qureshi S, Popat P, et al. (2015) Imaging in neuroblastoma: An update. Indian J Radiol Imaging 25: 129-136.
[Crossref], [Google Scholar], [Indexed]
- Brisse HJ, McCarville MB, Granata C, Krug KB, Gorges SWL, et al. (2011) Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology 261: 243-257.
[Crossref], [Google Scholar], [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences