Pneumonia Amidst the COVID-19 Pandemic: A Diagnostic Perplexity
Arushi Mohan, Abhinaya Shekhar ,Brunda MS and Prakash Doraiswamy
Department of Emergency Medicine, Junior Resident, Aster CMI Hospital, Bangalore, India
Published Date: 2022-05-30DOI10.36648/2471-8041.8.5.224
Arushi Mohan1*, Abhinaya Shekhar2, Brunda MS3 and Prakash Doraiswamy4
1Department of Emergency Medicine, Junior Resident, Aster CMI Hospital, Bangalore, India
2Department of Internal Medicine, Registrar, Aster CMI Hospital, Bangalore, India
3Department of Internal Medicine, Senior Consultant, Aster CMI Hospital, Bangalore, India
4Department of Anaesthesia and Critical Care Medicine, senior consultant Aster CMI Hospital, Bangalore, India
- *Corresponding Author:
- Arushi Mohan
Department of Emergency Medicine, Junior Resident, Aster CMI Hospital, Bangalore, India
E-mail:arushimohan@gmail.com
Received date: May 1, 2022, Manuscript No. IPMCRS-22-12864; Editor Assigned date: May 3, 2022, PreQC No. IPMCRS-22-12864 (PQ); Reviewed date: May 18, 2022, QC No. IPMCRS-22-12864; Revised date: May 24, 2022, Manuscript No. IPMCRS-22-12864(R); Published date: May 30, 2022, DOI: 10.36648/2471-8041.8.5.224
Citation: Brunda MS, Shekhar A, Mohan A (2022) Pneumonia Amidst the COVID-19 Pandemic: A Diagnostic Perplexity. Med Case Rep Vol.8 No.5:224
Abstract
Pulmonary nocardiosis is a subacute or chronic necrotizing pneumonia caused by aerobic actinomycetes of the genus nocardia and rare in immune-competent patients. Clinical diagnosis of pulmonary nocardiosis is difficult owing to the similarity of its presentation with other respiratory pathogens that include actinomycetes members and Mycobacterium tuberculosis. Laboratory diagnosis of human nocardiosis is plagued by the fact that a culture of Nocardia spp requires prolonged incubation periods for isolation which most laboratories fail to follow. The lack of clinical, laboratory and epidemiological data on the incidence of nocardiosis in humans undermines its significance as a potential pathogen. Here we present a case of pulmonary nocardiosis in an elderly gentleman who presented with similar features of Covid 19 who on further evaluation was found to have pulmonary nocardiosis. The patient had a past history of diabetes and myasthenia gravis on mycophenolate mofetil. Though nocardiosis is commonly seen in immunocompromised patients, the challenges we faced were diagnosing a case with similar radiological features of Covid 19 pneumonia and the difference in management strategies of the two.
Keywords
Nocardiosis; Myasthenia gravis; Immunosuppressed; COVID-19; Antibiotics; Resistance
Introduction
Pulmonary nocardiosis is a subacute or chronic necrotizing pneumonia caused by an anaerobic gram-positive bacillus that has branching hyphae. Of the various species of Nocardia, Nocardia asteroides are commonly involved. It affects patients that are immunosuppressed, making the management even more challenging [1].
Nocardiosis is an infection with increased morbidity and mortality and has the ability to cause localized or systemic suppurative disease in humans and animals [2].
The clinical diagnosis of pulmonary nocardiosis is difficult owing to the similarity of its presentation with other respiratory pathogens particularly those in the actinomycetes group and mycobacterium tuberculosis [3,4].
Our case highlights the diagnostic dilemma in the diagnosis of pneumonia in an immunosuppressed elderly male who went on to develop neutropenia in response to trimethoprim-sulphamethoxazole therapy which was initiated for nocardiosis.
Case Report
A 72-year-old male with type 2 diabetes mellitus, hypertension and myasthenia gravis presented to the emergency department with complaints of cough associated with sputum production, copious in amount, not blood-tinged. The patient also complained of breathlessness for ten days, classified as a grade three, by New York Heart Association (NYHA) grading. The patient also developed a fever which was of a high grade, intermittent type, and associated with chills. The patient had no complaints of chest pain or weight loss.
Past history
He had a recent exacerbation of myasthenia and a concomitant lower respiratory tract infection five days back. During this time, a High Resolution Computed Tomography (HRCT) chest scan was done to determine the involvement of the lungs, which was unremarkable and the patient was discharged on empiric antibiotic therapy. He had been diagnosed with Myasthenia Gravis for which he was started on prednisolone 30mg once a day, mycophenolate mofetil 500 mg twice a day, and pyridostigmine 60 mg 1-1-1-1 5 months back.
In the current visit, he was conscious and oriented requiring about 2 liters of oxygen, after which he maintained a saturation of 94%-95%. A bilateral wheeze was heard on the auscultation of his lungs. At this visit, an HRCT of the chest was done which revealed features suggestive of Coronavirus Disease-19 (COVID-19) pneumonia with a score of 15/25 as per the 25 point scoring system for COVID-19 and secondary infection. Gene expert for COVID 19 was negative. However, the CT findings were strongly suggestive of COVID-19 pneumonia and the Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) was positive for COVID-19.
The patient was admitted to the intensive care unit due to a drop in oxygen saturation and hypotension for which he was started on intravenous broad-spectrum antibiotics (meropenem and teicoplanin) along with supportive care.
Labs showed
Total count of 3.4K/ml, hemoglobin of 10.4mg/dl, and a platelet count of 134K/ml. Urine routine: Normal. Renal function test showed creatinine of 0.77mg/dL, sodium: 133mEq/L. In view of the respiratory symptoms, sputum analysis was done for gram stain, Acid-Fast Bacillus (AFB), Potassium Hydroxide (KOH) smear/fungal smear culture, and sensitivity, H1N1 swab, and flu panel which was negative.
On discussing with the neurologist regarding the possibility of an exacerbation of myasthenia, taking into account the immunosuppressed status and underlying infection. The dose of the steroids had been reduced and mycophenolate mofetil was stopped.
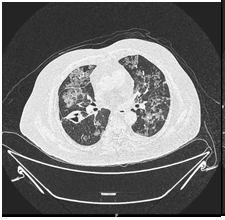
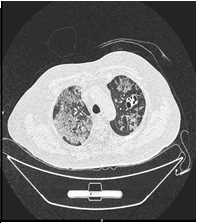
As the patient continued to deteriorate, a CT chest was done (Figure 1A), in comparison with the previous CT, there was evidence of an interval increase in the density of the subpleural region and peribronchial ground glass densities with interlobular septal thickening. These were mostly seen in the left upper lobes (now showing a crazy pavement pattern). There was an interval reduction in the solid component of consolidation located on the left upper and lower lobe. Nodular density in the right lower lobe measuring 9mm previously, a second time showed cavitation (Figure 1B).
Figure 1 B: Compared to the previous HRCT an interval increase in the density of the subpleural and peribronchial regions ground glass densities with interlobular septal thickening, seen in the upper lobes of the lung. An interval increase in a cavitary consolidation located on the left upper lobe. Nodular density in the right lower lobe measuring 9 mm which revealed a nodule previously now shows cavitation.
The patient continued to deteriorate despite receiving broad-spectrum antibiotics (meropenem and teicoplanin). Thus, a Bronchoscopy with bronchoalveolar lavage was considered.
| Parameters | Report |
|---|---|
| Acid-fast bacillus | Negative |
| Potassium hydroxide smear | Negative |
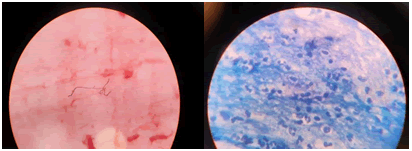
| Microscopy | Gram-positive branching filamentous structure was seen, High probability of nocardiosis |
| Gram stain | |
| Fungal cultures | Negative |
Table 1: Bronchoalveolar (BAL) analysis.
Meropenem and teicoplanin was stopped and intravenous imipenem with cilastatin 500mg 1-1-1-1 and tablet trimethoprim and sulphamethoxazole 2-2-2 were added. In view of this, complete blood count and renal function tests were monitored to capture any adverse events that may occur.
Magnetic Resonance Imaging (MRI) of the brain was done to look for disseminated nocardiosis. However, this was unremarkable.
While the patient was on the above medication he developed neutropenia: A potential drug-induced bone marrow suppression. Thus, mycophenolate mofetil and trimethoprim-sulfamethoxazole were withheld. The patient was then continued on imipenem. The patient developed asymptomatic hyponatremia (sodium: 120mEq/L) for which he was started on tab tolvaptan.
On consulting a hematologist for the same, granulocyte colony-stimulating factor (G-CSF) 300MU/day was given subcutaneously till a total count rise to above 4000/cumm (per cubic millimeter) was seen. After which he was restarted on Tab Trimethoprim and sulfamethoxazole 2-2-2-2.
The patient was discharged on Injection imipenem & cilastatin four times a day to complete a course of 15 days (11 days in the hospital). Trimethoprim and sulfamethoxazole were continued for 2 months.
Discussion
Pulmonary nocardiosis is the most common presentation of Nocardiosis. As evidenced by a retrospective study conducted on 25 patients over a 10-year period, 70% (18/25) had pulmonary nocardiosis [4,5]. However, diagnosing the condition can be challenging as its presentation is akin to pulmonary tuberculosis and other respiratory infections.
Most of the pulmonary infections begin as chest pain, cough breathlessness, bloody sputum, weakness, and loss of appetite which resembles any other pneumonia or even Tuberculosis [1,5]. Our patient had presented with cough and breathlessness and due to the pandemic was suspected of contracting SARS CoV2.
Predisposing factors can increase the chances of developing nocardiosis of which immunosuppressed states such as being diabetic, AIDS, post-organ transplant, chronic steroid use, Chronic Obstructive Pulmonary Disease (COPD). Of these, chronic glucocorticoid use in a sample of 35 patients demonstrated a risk of 64.5% [6]. This was the most common risk factor seen. Attributing steroids as a risk factor in case of nocardiosis was strengthened by a study done in Israel for over a decade on a sample of 75 patients 72% (n=54) in comparison to 18% (n=14) in other cases of pneumonia [7] Our patient was on steroids for Myasthenia Gravis and was also diagnosed with SARS-CoV-2 which is a relatively immunosuppressed state.
Physical exam reveals decreased breath sounds and occasional rales and crepitations. Radiological investigations may have quite nonspecific findings. The most common finding is nodules of size <30 mm. Consolidation, multiple nodules, cavity, mass, bronchial wall thickening, septal line thickening. Indeed, a more robust method of diagnosis is called for [8]. Thus, bronchoscopy with bronchoalveolar lavage and microbiological isolation of organism using gram stain and further Zeihl Neelsen stain is used to confirm the diagnosis (Figure 2). As was performed in our case, the chest X-ray with infiltrates, the HRCT chest (Figure 1 A, B), and bronchoscopy which further on Zeel Neelsen staining revealed branching elements of gram-positive rods.
The laboratory diagnosis of human nocardiosis is plagued by the fact that a culture of Nocardia species requires a prolonged incubation period for isolation which most laboratories fail to follow; it has a widespread distribution involving the lungs, brain, and skin [9] and the lack of clinical, laboratory and epidemiological data on the incidence of nocardiosis in humans undermines its significance as a potential pathogen.
The treatment of choice once a patient is diagnosed with nocardiosis is usually the sulfonamide group of drugs, particularly if the patient is immunosuppressed. Thus, in our patient, Bactrim (Trimethoprim-sulfamethoxazole) was considered. Other antibiotics that can be prescribed include amikacin, imipenem, cilastatin, meropenem, ceftriaxone [1,5]. However, there is increasing evidence of increasing resistance to the sulfonamide group of medication. Thus, newer drug combinations are being studied [10]. Imipenem and linezolid.
Imipenem can be used as a monotherapy or is used in combination with amkacin in nocardiosis. There was a complete microbiological and radiological reversal in such patients. 50% of patients had a resolution of symptoms after 2-3 cycles (each cycle consisted of therapy for 3 weeks followed by a repeat after 6 months). Others required a longer course but showed a 75% reduction in symptoms [11].
Thus, early recognition and effective therapy are imperative to achieve successful outcomes. Treatment is protracted particularly in patients that are immunosuppressed. Hence, careful consideration of antibiotics and their adverse effects should be monitored.
Conclusion
Pulmonary nocardiosis, an infection that masquerades the presentation of pulmonary infections makes the diagnosis challenging. Moreover, due to the rising use of steroids, there is an increased susceptibility of patients to develop this dreaded condition. A good grip on antimicrobial susceptibility and the adverse effects of antibiotics is salient.
References
- Rare diseases.Informa UK Limited.
- Rawat D, Rajasurya V, Chakraborty RK, Sharma S (2022) Nocardiosis. StatPearls, Treasure Island (FL): StatPearls Publishing.
- Kandi V (2015) Human nocardia infections: A review of pulmonary nocardiosis. Cureus 7: e304.
[Cross Ref], [Google Scholar], [Indexed] - Bansal Y, Singla N, Butta H, Aggarwal D, Gulati N, et al. (2021) Nocardia infections: Ten years experience from a tertiary health care center in north india (2007-2016). Infectious disorders drug targets 21: 445–451.
[Cross Ref], [Google Scholar], [Indexed] - Nocardiosis. Nocardiosis-An overview.
- Tomás RM, Villanueva RM, Calzada RS, Durantez DM, Tarazona JV, et al. (2007) Pulmonary nocardiosis: Risk factors and outcomes. Respirology 12: 394-400.
[Cross Ref], [Google Scholar], [Indexed] - Margalit I, Goldberg E, Ari YB, Ben-Zvi H, Shostak Y, et al.(2020) Clinical correlates of nocardiosis. Scientific Reports 10: 14272.
[Cross Ref], [Google Scholar], [Indexed] - Tsujimoto N, Saraya T, Kikuchi K, Takata S, Kurihara Y, et al. (2012) High-resolution CT findings of patients with pulmonary nocardiosis. J Thorac Dis 4: 577-582.
[Cross Ref], [Google Scholar], [Indexed] - Spelman D (2022) Clinical manifestations and diagnosis of nocardiosis.
- Brown-Elliott BA, Biehle J, Conville PS, Cohen S, Saubolle M, et al. (2012) Sulfonamide resistance in isolates of Nocardia spp. from a U.S. multicenter survey. J Clin Microbiol 50: 670–2.
[Cross Ref], [Google Scholar], [Indexed] - Ameen M, Arenas R, Mercado EV, Fernández R, Torres E, et al. (2010) Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol 62: 239-46.
[Cross Ref], [Google Scholar], [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences