Intradural Extramedullary Lumbar Spinal Metastasis with Unknown Primary Tumor Site: A Rare Case Report
Patrice LWY Sinaga* and Sabri Ibrahim
Department of Neurosurgery, Faculty of Medicine, Universitas Sumatera Utara Medan, Indonesia
- *Corresponding Author:
- Patrice LWY Sinaga
Department of Neurosurgery, Faculty of Medicine, Universitas Sumatera Utara Medan, Indonesia
Tel: 082166105050
E-mail: patricklwys@gmail.com
Received Date: December 03, 2021; Accepted Date: December 16, 2021; Published Date: December 23, 2021
Citation: Singa PLWY, Ibrahim S (2021) Intradural Extramedullary Lumbar Spinal Metastasis with Unknown Primary Tumor Site: A Rare Case Report Med Case Rep Vol.7 No.12
Abstract
Background
Intradural extramedullary spinal metastasis tumor in the lumbar region is an extremely rare condition. Neurological symptoms that arise from cauda equina compression are cauda equina syndrome.
Case Presentation
This study reports a case of a 41-years-old female with a history of progressive low back pain, paresthesia on lower extremities, mild paraparesis, and bladder symptoms. Patient had history surgery of breast and ovarian tumor without histopathology report. Lumbar spine MRI showed an intradural extramedullary solid lesion on the lumbar region. Gross total tumor removal was achieved. The histopathology report confirmed metastasis mucinous adenocarcinoma. The symptoms were improved postoperatively. Lumbar spine MRI postoperative show the tumor was removed completely.
Conclusion
The goals of surgery of intradural extramedullary spinal metastasis with unknown primary tumor site are tumor removal as much as possible, spinal cord and nerve root decompression, getting a definite diagnosis of tumor, and accepting quality of life.
Keywords
Intradural extramedullary; Metastasis; Lumbar; Unknown primary tumor site
Introduction
Spinal tumors are rare with around 5%-10% of all central nervous system tumors [1]. The spinal vertebral column is a relatively common site of metastasis tumor. Otherwise, the intradural extramedullary site of the metastasis tumor is an extremely rare condition [2]. In most cases, the solid metastasis tumor in the intradural extramedullary spinal is located on a nerve root. It makes difficulty differentiation from a nerve sheath tumor [3]. Few studies report metastasis tumor to cauda equina which origins from kidney, lung, and breast cancer [2]. Intradural extramedullary spinal metastases from various types of cancers are uncommon, and they are found at autopsy in less than 5% of all cancer patients [4].
Neurological symptoms in cancer patients arise from cauda equina compression after secondary deposit of metastasis tumor on the lumbar [5]. The neoplasm that metastasizes to the cauda equina have symptoms like low back pain, bilateral sciatica, motor weakness of the lower extremities, and rectal bladder sphincter dysfunction. These clinical symptoms were known as cauda equina syndrome. It is the second most common neurological complication of cancer after brain metastase. Metastatic tumors in intradural extramedullary can arise from Central Nervous System (CNS) elements or extra-Central Nervous System (CNS) elements. Tumors from the extra CNS that can be metastases of the intradural extramedullary are cancers from lung, breast, melanoma, lymphoma, and leukemia. Tumors from CNS elements that metastasis to the spinal subarachnoidofintraduralextramedullaryareglioblastoma,anaplasticastrocytoma,medulloblastoma, pineal body tumor and choroid plexus tumor [6].
Magnetic Resonance Imaging (MRI) assessment frequently helps identification of the tumor, while their features are not always pathognomonic. MRI examination is the standard imaging technique for confirming the presence of metastatic disease, even when the results of cerebrospinal fluid analysis are negative [7].
The type of primary tumor was reported as one of the most powerful prognostic factors [8]. To select the best treatment option for the patients, it is important to identify the primary tumor type [9]. There are many factors influencing the survival prognosis, such as identification of tumor histology, overall functional status, neurological status, and the overall burden of the disease [8].
This study presents a unique case report of metastatic mucinous adenocarcinoma to the intradural extramedullary space at the lumbar region without known primary tumor site. The symptoms and signs of advanced cancer, such as fatigue, lumps, swelling, lymph node, and unintended weight loss are unidentified.
Case Report
The case report presents a case of a 41-years-old female with a history of low back pain for 1 year before she presented at our facility. She had come to the neurologist facility after she complained of progressively low back pain. She also experienced paresthesia on both lower extremities for 1 year. She had mild bladder disorder for 3 months, with the feeling of incomplete bladder emptying.
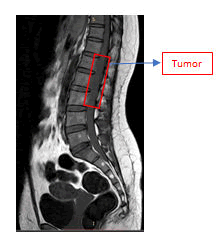
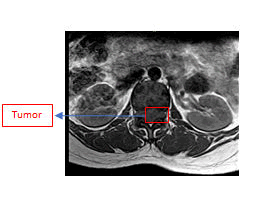
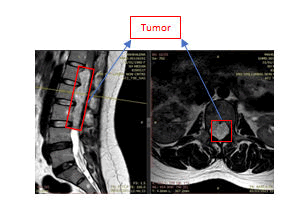
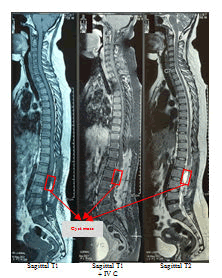
Physical examination was normal. She was conscious. She has linear incision scar on the upper outer site of the right breast after surgery of breast tumor removal in five years ago and Pfannenstiel incision scar on the lower abdomen quadrant after surgery of ovarium cyst resection in 2 years ago. There are not documents of histopathology examinations of both tumors. There are not swellings of lymph nodes or organomegaly or unexplained weight loss. Breast and abdominal examination are unremarkable. Specifically, the strengths of L4 and L5 myotomes of both lower motoric extremities were 4, otherwise the strength of L2, L3, and S1 myotomes of both lower extremities were 5. The meningeal signs are unremarkable. Routine laboratory investigations showed mild anemia (Hb was 9,3 gr/ml), while others were normal. Lumbar spine MRI without contrast showed a intradural extramedullary solid lesion from thoracal 12 to lumbar 3. Unfortunately, the enhancement of the tumor was not identified because the lumbar spine MRI with contrast did not work and are not underwent in this study of the patient. The tumor effected compression of the conus medullaris to lateral. There is a compression effect of bilateral neural foramen from lumbar 2 to lumbar 4 (Figures 1, 2, and 3). A working diagnosis of an intradural extramedullary lesion was made, and the patient was scheduled for surgery.
T1W1 sagittal sequence of MRI spine lumbar shows hypointense of intradural extramedullary tumor from Th12-L3.
T1W1 axial sequence MRI spine lumbar shows hypointense of intradural extramedullary tumor
T2W2 sagittal and axial sequences of MRI spine lumbar shows homogenous enhancement of intradural extramedullary tumor from Th12-L3.
Gross total removal of the tumor with maximum function preservation of the nerve roots of cauda equina and conus medullaris are the goal of surgery to be achieved since the strength of both lower extremities were high. After general anesthesia was done, the patient was positioned prone. The skin incision was prepared by washing the area of the incision, marking the skin, and then dropping with povidone-iodine.
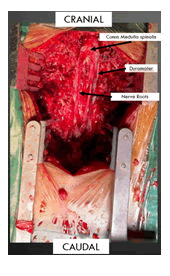
The skin incision was made vertically in the midline from thoracal 11- lumbar 4, and hemostasis was applied with cauterization. Subperiosteal dissection of the spinous process and lamina was done by electrocautery and Cobb elevator. Spinous process Th 12-L3 was removed by Horsley bone cutter and double-action rongeur. Laminectomy at Th12-L3 was completed by high-speed drill and Kerrison rongeurs. Careful removal of the ligamentum flavum (flavectomy) can be done with a rongeur (Figure 4). After flavectomy, the dura mater was exposed. Dura mater was bulging and tense. An initial dural opening is performed using a number 15 blade. Metzenbaum scissors were used to complete the dural opening. To maintain wide intradural exposure, 4-0 nurolon tenting sutures are placed in the dura. The reddish-dark solid tumor was exposed. Gross total tumor removal was done completely with preservation of nerve roots and conus medullaris (Figures 5 and 6). After meticulous hemostasis, the dura was closed with 4-0 nurolon suture. Plug of fat was applied over the suture line to prevent cerebrospinal fluid leak. The skin was closed in layers by placing a subgaleal drain tube.
Laminectomy and flavectomy from thoracal 12-lumbar 3 were done to expose duramater.
Gross tumor removal was done.

Figure 6: Tumor
Pieces of the tumor.
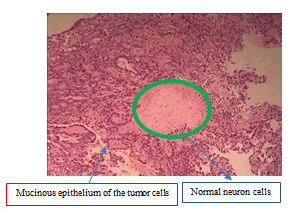
Surprisingly, the pathology report confirmed metastasis mucinous adenocarcinoma. There are mucinous epithelial cancer cells under microscopic examination (Figure 7 and 8). Postoperatively, the paresthesia was disappeared and the strength of L4 and L5 myotomes was improved gradually and meningeal signs are unremarkable. Low back pain and bladder symptoms were also improved postoperatively.
Mucinous adenocarcinoma (Hematoxylin and Eosin (HE): original magnification, x200).
Mucinous adenocarcinoma (Hematoxylin and Eosin (HE): original magnification, x200). Green circle shows normal neuron cells.
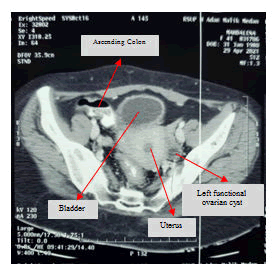
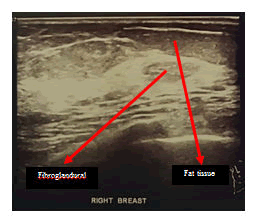
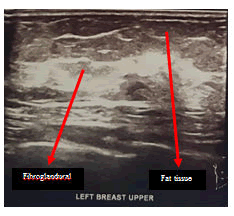
Lumbar spine MRI postoperative shows total tumor removal was achieved. There were no signs of intramedullary metastatic spread or signs of cerebrospinal fluid blockage. There are no signs of corresponding to diffuse leptomeningeal spread of metastases. There is a round-shaped cystic lesion intradurally at lumbar 2. We doubt it as a tumor because the lesion is not enhancing contrast agent (figure 9). The patient underwent upper lower abdominal CT scan IV contrast and breast ultrasonography (USG) to evaluate suspected pathologic mass. The abdominal CT scan IV contrast showed a round circumscribed hipodens mass in the left adnexa that suggest a physiologic cyst of left ovarium (figure 10). There is no pathologic mass in intraabdominal. The breast USG showed no pathologic mass in in both breasts which showed normal homogenous fibroglandular and fat tissues in both breasts (Figures 11 and 12).
MRI spine lumbar IV Contrast postoperative shows total tumor removal with a cystic lesion in the lumbar 2.
Axial lower abdominal axial CT scan IV Contrast showed a left functional ovarian cyst.
Right breast USG shown opathologic focal lesion. Fibro glandular and fat tissues are homogeneous.
Left breast USG shown opathologic focal lesion.Fibro glandular and fat tissues are homogeneous.
Discussion
Intradural metastases are a relatively rare locations compared to vertebral column metastases [2]. Intradural metastases are a severe form of systemic cancer manifestation [10]. Metastasis to the intradural extramedullary space is an exceedingly rare condition [4]. The majority of metastasis cases occur in the 6th decade of life. There are no significant differences in gender in this disease according to the existing literature [10].
All carcinoma types seem to be capable of spreading intradurally. The vast majority of metastasis cases in both intradural extramedullary space and intramedullary are from lung and breast carcinoma [10]. Other study reports the most of the cancer that metastasis in intradural is the lung (40-85%), followed by breast cancer and renal cell cancer [2]. The series studies showed metastases were equally distributed along the cervical, thoracic, and lumbar spine. The majority of intradural extramedullary spinal metastases are reported to be located in the lumbar spine. An explanation of this phenomenon might be the lower CSF flow velocity in the lumbar spine compared to the cervical and thoracic segment, causing a higher probability for tumor cells to settle [10].
Our patient’s case is considered quite rare because the initial syndromes were low back and lower extremity pain presenting as cauda equina syndrome without associated signs and symptoms of advanced cancer. According to one of the reports, as in our patient’s case, MRI of the intradural extramedullary tumor metastasis in the cauda equina appeared similar to that intradural extramedullary nerve sheath tumor or myxopapillary ependymoma or Ewing tumor. Diagnosis by MRI alone was difficult because the primary site of cancer was unknown [11].
MRI replaced computed tomography myelography as the imaging of choice in diagnosing intradural extramedullary spinal cord lesions. Unfortunately, intradural extramedullary spinal metastasis is unable to distinguish from primary spinal cord tumors or other non-neoplastic lesions [12]. Therefore, we suggested the patient had primary spinal cord tumor preoperatively based on clinical manifestation, imaging, and unidentified advanced cancer symptoms.
There are many factors to playing roles in the routes of spread for intradural extramedullary lesions. Further research is required to identify factors that predispose neoplasms to metastasize via specific routes to the intradural extramedullary space. As an example, non-small cell lung cancer has been shown to exhibit a propensity for CNS involvement after mutations in the ALK gene [13].
One or combinations of 5 mechanisms are proposed as the way how neoplastic cells can reach the intradural extramedullary space. They are Batson’s venous plexus, perineural lymphatics, seeding from local invasion adjacent to the dura, spread from the subarachnoid space in so-called “drop metastasis” or leptomeningeal fashion, and hematogenous spread via the arterial system. Batson’s plexus spreading has been implicated in a large fraction of case reports as well [14].
Intradural extramedullary spinal metastasis in the lumbar region originating from a primary tumor outside the central nervous system is exceedingly rare and usually discovered secondary to symptoms of compression of nerve roots, cord, conus, or cauda equine. Most cancer metastasi in the intradural extramedullary space involving the cauda equina are prostate, kidney, thyroid, lung, and breast [14]. The unique case in this study presents intradural extramedullary spinal metastasis in the lumbar region with history of surgery, resection of ovarian cyst, and breast tumor in 2 and 5 years ago respectively. The histopathology examination of the surgery is not documented.
Cauda equina syndrome is the majority affected by primary neurologic tumors such glioma and meningioma such as isolated cauda equina tumors or intradural extramedullary lumbar region tumor [15]. Otherwise, intradural extramedullary cauda equina metastatic tumors from outside the central nervous system are very rare. Renal cell carcinoma was the most common cancer that metastasizes to cauda equina in the reported cases [16].
The primary role of surgery is the preservation of neurological function, without increasing disease-free survival [10]. Surgical treatment for the intradural lesions appears to be the mainstay for tumors that present acutely with neurological deficits. It is desirable to decompress the neural elements and relieve the compressive pathology by complete resection of the intradural extramedullary lesion. In the vast majority of cases, pain relief and or motor power are improved postoperatively even when simple debulking or subtotal resection is performed [14]. Other studies showed an increased survival rate and significant improvement of pain and neurological deficits in those patients with intradural extramedullary spinal metastasis treated surgically compared to those patients with intradural extramedullary spinal metastasis treated conservatively [10]. The patient in this study was quite satisfied with the surgery because her low back and lower extremity pain was relieved substantially. Surgery treatment may be a favorable option to improve the quality of life in such cases, even if the patient’s prognosis is not good [2].
Surgery has better survival compared to conservative treatment with a median survival time of 9,4 vs 5 months and 7,4 vs 2,6 months with and without surgery, respectively. The major benefit of surgical treatment is a rapid and significant improvement of pain and neurological deficits [10].
A laminectomy decompression surgery mostly is necessary in cauda equina syndrome cases. Most of the surgeries can improve the syndrome even it could’t help to improve the survival time and avoid the complication of spinal fluid leak, hematoma, postoperative infections, and failure to respect the tumor completely [16]. Our patient underwent laminectomy decompression with gross total resection of her intradural extramedullary spinal metastasis resulting in improved neurological status. The tumor has defined borders that enable to be removed completely.
Histopathologyexaminationofthepatientinthiscaseshowsametastasismucinousadenocarcinoma with unknown primary tumor site. After getting the confirmation result of the histopathology study, the patient underwent two tools, screening of the breast and intraabdominal. The studies show no pathologic findings.
Mucinous adenocarcinomas are a group of malignant tumors that originate from epithelial tissue and are characterized by abnormal mucus secretion. According to WHO classification, mucinous adenocarcinoma has characteristics as the cells secrete abundant extracellular mucins involving more than 50% of the tumor volume [17]. The most majority site finding of mucinous adenocarcinoma is in colorectal cancer (approximately 10–15% of all colorectal cancers), followed by other glandular organs, such as the breast, stomach, ovary, and lung [18]. The most common histological finding of unknown primary site is adenocarcinoma followed by squamous cell carcinoma and neuroectodermal carcinoma [19].
Conclusion
It is difficult to clarify the intradural extramedullary tumors are benign or malignant on the basis of MRI findings alone. Consideration of the possibility of metastasis is important before planning surgery to extirpate tumors from the intradural cauda equina. Surgical treatment is preferred in intradural extramedullary spinal metastasis with unknown primary tumor site, although the prognosis for such metastatic tumors in the lumbar region is not good. The goals of surgery are tumor removal as much as possible with functional preservation, spinal cord and nerve root decompression, getting a definite diagnosis of tumor, and accepting quality of life.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
We are grateful to all of the staff of Department of Neurosurgery, Faculty of Medicine, Universitas Sumatera Utara Medan.
References
- Özkan N, Jabbarli R, Wrede KH, Sariaslan Z, Stein KP, et al. (2015) Surgical management of intradural spinal cord tumors in children and young adults: a single-center experience with 50 patients. Surgical Neurology International 6:661-667
- Koyama K, Takahashi H, Inoue M, Okawa A, Nakajima A, et al. (2019) Intradural Metastasis to the Cauda Equina Found as the Initial Presentation of Breast Cancer: A Case Report. J Med Case Rep 13:1-4
- Jung YJ, Kim SW, Chang CH, Cho SH (2005) Intradural Extramedullary Non-infiltrated Solitary Metastaic Tumor. J Korean Neurosurg 37:466-468
- Anand SK, Garling RJ, Johns J, Shah M, Chamiraju P (2020) Intradural extramedullary spinal metastases from uteri carcinoma: A case report. Surgical Neurology International:11
- Mehrotra R, Sharma K (2002) Intradural Extramedullary Spinal Metastasis from an Ovarian Carcinoma. Indian J Cancer 39:157
- Lin CL, Wu KA, Chang JL, Lo HC (2010) Extramedullary-intradural spinal metastasis of small cell lung cancer causing cauda equina syndrome. Am J Med Sci 339:192-194
- Koeller KK, Shih RY (2019) Intradural Extramedullary Spinal Neoplasms: Radiologic-Pathologic Correlation. Radiographics 39:468-490
- Rose PS, Buchowski JM (2011) Metastatic disease in the thoracic and lumbar spine: evaluation and management. J Am Acad Orthop Surg 19:37-48
- Kataoka M, Kunisada T, Tanaka M, Takeda K, Itani S, et al. (2012) Statistical analysis of prognostic factors for survival in patients with spinal metastasis. Acta Med Okayama 66: 213-219
- Wostrack M, Pape H, Kreutzer J, Ringel F, Meyer B, et al. (2012) Surgical treatment of spinal intradural carcinoma metastases. Acta Neurochir 154:349-357
- Kotil K, Kilinc BM, Bilge T (2007) Spinal metastasis of occult lung carcinoma causing cauda equina syndrome. J Clin Neurosci 14:372–375
- Akhavan A, Mehrabaniyan MR, Jarahi M, Navabii H (2012) Intradural extramedullary metastasis from papillary carcinoma of thyroid. BMJ Case Rep:bcr0220125801
- Karsy M, Guan J, Sivakumar W, Neil JA, Schmidt MH, et al. (2015) The genetic basis of intradural spinal tumors and its impact on clinical treatment. Neurosurg Focus 39:E3
- Land CF, Bowden BD, Morpeth BG, DeVine JG (2019) Intradural Extramedullary Metastasis: A Review of Literature and Case Report. Spinal Cord Ser Cases 5:1-6
- Kim DY, Lee JK, Moon SJ, Kim SC, Kim CS (2009) Intradural spinal metastasis to the cauda equina in renal cell carcinoma: a case report and review of the literature. Spine (Phila Pa 1976) 34: 892-895
- Xiong J, Zhang P (2015) Cauda equina syndrome caused by isolated spinal extramedullary-intradural cauda equina metastasis is the primary symptom of small cell lung cancer: a case report and review of the literatrure. Int J Clin Exp Med 8:10044–10050
- Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. World Health Organization
- Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID (2014) Insight into mucinous colorectal carcinoma: clues from etiology. Ann Surg Oncol 21:2963–2970
- Paholpak P, Sirichativapee W, Wisanuyotin T, Kosuwon W, Jeeravipoolvarn P (2012) Prevalence of known and unknown primary tumor sites in spinal metastasis patients. Open Orthop J 6:440-444

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences