Efficiency of Nivolumab Considering Size of Tumor Thrombus in a Case of Metastatic Clear Cell Renal Cell Carcinoma with an Extensive Right Atrial Tumor Thrombus: A Case Report
Laura Bender, Justine Gantzer, Jean Caudrelier, François Somme, Jean Emmanuel Kurtz and Philippe Barthélémy
Laura Bender1*, Justine Gantzer1, Jean Caudrelier2, François Somme3, Jean Emmanuel Kurtz1 and Philippe Barthélémy1
1Department of Oncology, University Hospital of Hautepierre, Strasbourg, France
2Department of Interventional Radiology, University Hospital, Strasbourg, France
3Department of Nuclear Medicine, University Hospital Hautepierre, Strasbourg, France
- *Corresponding Author:
- Laura Bender
Department of Oncology, University Hospital of Hautepierre, Strasbourg, France
Tel: + 33 (0)3 88 12 76 65
Fax: + 33(0)3 88 12 54 89
E-mail: laura_2708@hotmail.fr
Received date: March 25, 2019; Accepted date: April 15, 2019; Published date: April 15, 2019
Citation: Bender L, Gantzer J, Caudrelier J, Somme F, Kurtz JE (2019) Efficiency of Nivolumab Considering Size of Tumor Thrombus in a Case of Metastatic Clear Cell Renal Cell Carcinoma with an Extensive Right Atrial Tumor Thrombus: A Case Report. Med Case Rep Vol.5 No.2:98.
Abstract
Renal-cell carcinoma is frequently associated with tumor thrombus at initial diagnosis. The tumor thrombus is associated with a pejorative survival prognosis. Targeted molecular therapies such as sunitinib have a modest impact on size and level of tumor thrombus. Actually, nivolumab plus ipilimumab combination is the new standard of care as first line for metastatic renal carcinoma. We reported a case of an 87-year old man with a metastatic renal-cell carcinoma and a tumor thrombus level IV. The patient was treated with nivolumab as first line. After seven months of treatment, there was a partial regression of the tumor thrombus size without impact of the tumor thrombus level. To our knowledge, we reported for the first time the positive impact of checkpoint inhibitor on tumor thrombus size in a case of metastatic renal-cell carcinoma with an extensive tumor thrombus.
Keywords
A clear-cell renal-cell carcinoma; Immunotherapy; Nivolumab; Tumor thrombus
Introduction
Renal-cell carcinoma (RCC) is the ninth most common cancer with 338,000 new cases and 143,000 deaths in Europe in 2012 [1]. Venous tumor thrombus (TT) is frequently reported in RCC with a rate of 4 to 15% [2]. According to the Novick classification, tumor thrombus extension is classed into four levels; level I: only renal vein (RV) involvement, level II: inferior vena cava (IVC) under 2 centimeter (cm) above RV, level III: IVC above hepatic veins and below diaphragm and level IV: above diaphragm. There is a lack of data considering the evaluation of TT under targeted therapy. However, a potential impact of targeted therapy on RCC tumor thrombus was noticed. Two retrospectives studies, including 39 patients, described a modest impact of targeted molecular therapies on the size and the level of TT in RCC [2,3]. Checkpoint inhibitors (mainly anti PD(L)1 and anti CTLA4) led to a revolution in RCC treatment in second and third line [4] as well as more recently in first line 5). Immunooncology (IO) therapies improved overall survival. In first line, ipilimumab-nivolumab combination showed a response rate of 42% (vs 27%, p<0.001), a median progression free survival time of 11.6 months (vs 8.4 months, p=0.03), as well as a 18 months overall survival (OS) rate of 75% as compared to 60% under sunitinib (5). In second and third line, nivolumab showed a response rate of 25% (vs 5%, p<0.001) and a median OS time of 25 months compared to 19.6 months under everolimus. Trials are currently in progress to study the impact on survival of checkpoint inhibitors in adjuvant (RAMPART trial) and neoadjuvant setting (PROSPER RCC trial) for patients with localized renal carcinoma. However, no data are available on the potential efficacy of checkpoint inhibitor on a TT. We report here to our knowledge, the first results of the potential role of a checkpoint inhibitor on the size and the level of tumor thrombus in case of metastatic RCC (mRCC).
Case Presentation
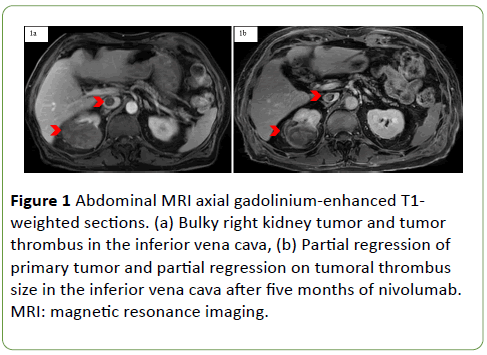
A 87-year-old man with an Eastern Cooperative Oncology Group score of 1 and no previous medical history, presented in September 2017 a diffuse pruritus. In March 2018, an abdominal magnetic resonance imaging (MRI) was performed and found a bulky tumor (110 × 85 mm) in the posterior and lower pole of the right kidney with an inferior vena cava TT (Figure 1).
Figure 1: Abdominal MRI axial gadolinium-enhanced T1- weighted sections. (a) Bulky right kidney tumor and tumor thrombus in the inferior vena cava, (b) Partial regression of primary tumor and partial regression on tumoral thrombus size in the inferior vena cava after five months of nivolumab. MRI: magnetic resonance imaging.
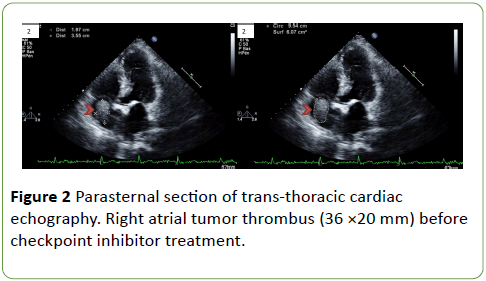
Thoracic computed tomography (CT) was performed and revealed multiple lung metastases [Response Evaluation Criteria in Solid Tumors (RECIST) 1.1: 122.5 mm]. Transthoracic cardiac-echography found a TT (36 × 20 mm) in the right atrium (Figure 2).
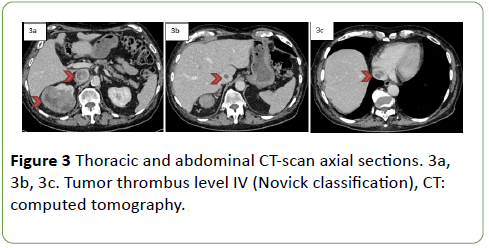
A whole-body CT confirmed the right kidney tumor with pulmonary metastasis and the TT classed level IV (Figures 3a, 3b, 3c).
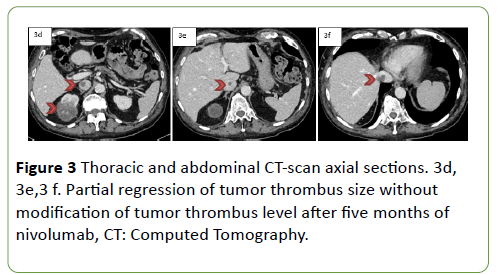
According to the European guidelines, on April 2018 a percutaneous biopsy of the primary tumor was performed. The diagnosis made was a clear-cell RCC with necrosis and less than 5% of a rhabdoid component. The patient was treated with nivolumab, a checkpoint inhibitor anti PD-1. Treatment was initiated in June 2018, and after five months of nivolumab, a thoracic CT showed a partial regression of the pulmonary metastasis. An abdominal MRI found a partial size regression of the primary right kidney tumor (RECIST 1.1: 82 mm versus 122.5 mm; -67%) with a positive impact on the tumor thrombus size (Figure 1b). A thoracic and abdominal CT confirmed that the checkpoint inhibitor anti-PD-1 had induced a partial regression on the tumor thrombus size without an impact of TT level (Figure 3c, 3d, 3e).
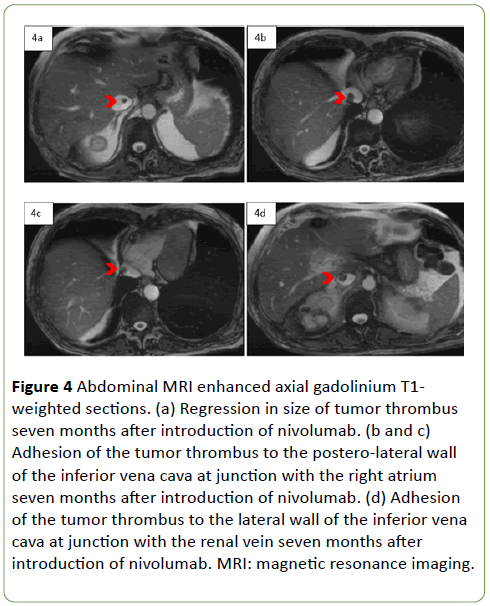
Seven months after the beginning of nivolumab, after multidisciplinary discussion, a nephrectomy with a thrombectomy was proposed. The abdominal MRI made to assess the size of the tumor thrombus found a regression (Figure 4a), but the thrombus was still adhering to the lateral wall of the inferior vena cava at junction with the right atrium (Figures 4b and 4c) and with the renal vein (Figure 4d).
Figure 4: Abdominal MRI enhanced axial gadolinium T1- weighted sections. (a) Regression in size of tumor thrombus seven months after introduction of nivolumab. (b and c) Adhesion of the tumor thrombus to the postero-lateral wall of the inferior vena cava at junction with the right atrium seven months after introduction of nivolumab. (d) Adhesion of the tumor thrombus to the lateral wall of the inferior vena cava at junction with the renal vein seven months after introduction of nivolumab. MRI: magnetic resonance imaging.
Therefore, the surgical treatment was not performed due to post-operative risks of complications and patient is still treated with nivolumab.
Discussion
To our knowledge, this is the first reported case, which evaluates the impact of an anti PD 1 antibody on the TT size for a patient with a stage IV RCC. Previous studies suggest a role of systemic treatment on TT; indeed Bigot et al. described the impact of TKI (sunitinib and sorafenib) on tumor thrombus size and level for 14 patients with localized renal carcinoma. Among the 14 patients included in the study, 6 (43%) had a decrease, 6 (43%) had no change and 2 (14%) had an increase of the thrombus size. The tumor thrombus level did not change for 12 (85%) patients [2,5]. Currently no data are yet available on a potential activity of immunotherapy agents on TT. Large randomize phase III trials did not mention the activity on such TT. As immunotherapy agents enter now in the neoadjuvant setting, it is important to know if patients with TT can expect a potential activity of IO on the TT and if such neoadjuvant approach can modify the surgery. Our case suggested a potential activity of nivolumab on TT. However, surgical approach was not modified, as the treatment did not downstage the TT level. Lopez et al. analyzed the expression of PD-L1 in primary tumor and venous TT in 39 cases of RCC. Twenty-two (56.4%) patients had a positive PD-L1 expression in the primary tumor. Among them, 18 patients (81.8%) did not express PD L1 on the TT suggesting a high heterogeneity of PD L1 expression. This result might explain in our case the partial impact of the anti PD-1 checkpoint inhibitor on the size of TT but the lack of impact on the TT level [6]. Further trials should be done to assess the activity of immunotherapy agents on TT. Recently IO and tyrosine kinase inhibitor (TKI) combinations showed a better overall survival in the first line metastatic setting compared to sunitinib [7]. These therapeutic options could have an impact on the tumor thrombus size and level. Future studies are still needed to evaluate the impact of IO TKI combinations on TT. In our case, the patient presented a partial response to nivolumab on the lung metastases as well as on the primary tumor. Recent checkmate 214 study data suggested that among partial or complete responder, most patients are still in response after a follow up of 30 months (Long term follow up data checkmate 214, oral presentation on 16 Feb 2019 at the American Society of Clinical Oncology 2019 Genitourinary Cancers Symposium in San Francisco, CA, US.). Furthermore, in the multivariate analysis of Haferkamp et al. prospective study, including 134 patients with RCC and inferior vena cava thrombus, the TT level was an poor prognosis factor (HR 1.77 IC 95% 1.05-2.98, p=0.0323) [8]. Tilki et al. confirmed the TT level as a poor prognosis factor (p<0.001) in their retrospective study, which included 1774 patients with RCC, and TT treated by surgery [9]. Moreover, Sidana et al. reported a 48% 5-year OS rate after surgery for patients with RCC and renal TT compared to the only 13% for patients with an extensive IVC thrombus (above diaphragm) [10].
Conclusion
Due to the unfavorable prognosis of TT in RCC, future studies are needed to determine the impact of the different checkpoint inhibitors or IO TKI combinations on the size and level of TT for patients with mRCC. Further clinical trials should also assess the potential role of primary tumor surgery for the patients treated by checkpoint inhibitor for mRCC with TT, in complete response on metastatic lesions.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Authors’ Contributions
L.B. and J.G. analyzed the patient’s data and contributed in the writing of the manuscript.
J.C. and F.S. designed the figures
P.B. and J-E.K. drafted the manuscript and underwent a critical revision.
References
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F (2015) International variations and trends in renal cell carcinoma. Incidence and Mortality. Eur Urol 67: 519-530.
- Bigot P, Fardoun T, Bernhard JC, Xylinas E, Berger J, et al. (2019) Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: Is it useful? World J Urol 32: 109-114.
- Cost NG, Delacroix SE, Sleeper JP, Smith PJ, Youssef RF, et al. (2011) The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 59: 912-918.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, et al. (2015) Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. NEJM 373: 1803–1813.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, et al. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. NEJM 378: 1277-1290.
- López JI, Pulido R, Lawrie CH, Angulo JC (2018) Loss of PD-L1 (SP-142) expression characterizes renal vein tumor thrombus microenvironment in clear cell renal cell carcinoma. Ann Diagn Pathol 34: 89-93.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, et al. (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. NEJM.
- Haferkamp A, Bastian PJ, Jakobi H, Pritsch M, Pfitzenmaier J, et al. (2007) Renal Cell Carcinoma with Tumor Thrombus Extension into the Vena Cava: Prospective Long-Term Followup. J Urol 177: 1703-1708.
- Tilki D, Nguyen HG, Dall’Era MA, Bertini R, Carballido JA, et al. (2014) Impact of Histologic Subtype on Cancer-specific Survival in Patients with Renal Cell Carcinoma and Tumor Thrombus. Euro Urol 66: 577-583.
- Sidana A, Goyal J, Aggarwal P, Verma P, Rodriguez R (2012) Determinants of outcomes after resection of renal cell carcinoma with venous involvement. Int Urol Nephrol 44: 1671-1679.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences