Decellularized Bovine Bone Graft for Zygomatic Bone Reconstruction
Karalashvili L, Chichua N, Menabde G, Atskvereli L, Grdzelidze T, Machavariani A, Zurmukhtashvili M, Gogilashvili K, Kakabadze Z and Chichua Z
DOI10.21767/2471-8041.100087
1Department of Clinical Anatomy, Tbilisi State Medical University, Tbilisi, Georgia
2Departments of Stomatology and Maxillofacial Surgery, Tbilisi State Universities, Tbilisi, Georgia
3Institute of Medical Research, Ilia State University, Tbilisi, Georgia
4Departments of Maxillofacial Surgery, S. Khechinashvili University Clinics, Tbilisi, Georgia
- *Corresponding Author:
- Lia Karalashvili
Department of Clinical Anatomy
Tbilisi State Medical University
33 Vazha-Pshavela Avenue, 0186
Tbilisi, Georgia
Tel: +995598703772
E-mail: likakaralashvili@gmail.com
Received Date: November 14, 2017 Accepted Date: November 26, 2017 Published Date: November 28, 2017
Citation: Karalashvili L, Chichua N, Menabde G, Atskvereli L, Grdzelidze T (2017) Decellularized Bovine Bone Graft for Zygomatic Bone Reconstruction. Med Case Rep Vol.4 No.1:52. doi: 10.21767/2471-8041.100087
Abstract
Reconstruction of large size maxillofacial bone defects following trauma or tumor resection still represents a challenging task for surgeons. Various types of bone materials are used as the grafts for the reconstruction of bone defect: auto, allo, xeno and alloplastic bones. In recent years, using the methods of decellularization has enabled to obtain the natural,biocompatible threedimensional xenograft for the reconstruction of bone defects. Removing DNA from the bone matrix is critical because this DNA may stimulate immune reactions by activating cytokine production and B-cell immunoglobulin secretion after transplantation. For the large maxillofacial bone reconstruction procedures, the decellularized bovine bone graft has proven to be effective, easy to handle and biocompatible, providing excellent final aesthetic and functional results. However, more animal and clinical studies are required to further assess the osteogenic activity and integration of decellularized bone grafts with the native bone.
Keywords
Zygomatic bone; Decellularized bovine bone graft; Autogenous bone; Maxillofacial surgery
Introduction
Reconstruction of large size maxillofacial bone defects following trauma or tumor resection still represents a challenging task for surgeons. The requirements for successful bone repair relies on such factors as types of trauma, bone defect size, biocompatibility of bone graft, its size, shape, structure, and biomechanical characteristics, methods of graft fixation, ability to support vascular in-growth and meet the mechanical requirements for bone regeneration [1]. Various types of bone materials are used as the grafts for the reconstruction of bone defect: auto, allo, xeno and alloplastic bones [2]. Vascularized autogenous bone is still being considered to be the gold standard for the reconstruction of bone defects since it has optimal osteoinductive, osteoconductive and osseointegration properties [3]; however, harvesting autologous bone graft is associated with morbidity and a number of complications [4]. Also, large size free autologous bone graft may be subjected to resorption [5]. Allografts and xenografts have osteoinductive and osteoconductive characteristics but lack the osteogenic properties of autografts [6,7]. Allogeneic and xenogeneic bone grafts have a risk of transmitting various diseases [8,9]. The synthetic bone grafts are unable to provide full bone regeneration. This is due to insufficient vascularization, weak osteoconductivity, and inadequacy of bone formation, lower mechanical resistance and stability of the graft [10].
Considering these challenges, the advancements in the methods of tissue engineering in the last decade enabled the creation of new scaffolds for bone grafts aiming to decrease the disadvantages of traditional grafts [11].
The aim of the present study was to create the threedimensional bone graft from the decellularized bovine femoral bone for the reconstruction of large size maxillofacial bone defects and its use in the clinical practice.
Patient and Methods
Creation of the decellularized bovine bone graft
Fresh samples of bovine femur bones were collected from a slaughterhouse (Tbilisi, Georgia) 3–4 h subsequent to slaughter. Following bacteriological analysis, the bone was separated from the soft tissue. The femur bones were rinsed in running water for 1 h and cut with a saw (JG210 Bone Cutting machine; Shandong China Coal Industrial & Mining Supplies Group Co., Ltd., Shandong, China) into bone fragments of 15 × 4 × 2 cm. Bone fragments were placed in deionized water solution containing 5,000 units/ml heparin (Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) for 24 h to remove blood components present in the bone.
Subsequently, the bone fragments were rinsed with 800 ml 0.9% saline solution and frozen at −80°C for at least 12 h (fragments were fully submerged in the solution). Sodium dodecyl sulfate (SDS; Sigma-Aldrich; EMD Millipore) and Triton X-100 (Sigma-Aldrich; EMD Millipore) has been used for the decellularization of the bovine bone according to the protocol that was described earlier [12]. In short, the frozen fragments of bone were thawed at 4°C and rinsed with phosphatebuffered saline (PBS; Sigma-Aldrich; EMD Millipore) prior to sequential washes in 0.01, 0.1 and 1% SDS solution for 72 h.
Bone fragments were rinsed with distilled H2O for 45 min, followed by 1% Triton X-100 for 2 h to remove the remaining SDS. Following Triton X-100 treatment, the decellularized bone fragments were rinsed with PBS for 4 h. The scaffolds were placed in a stirrer and rinsed with a solution containing chloroform and ethanol, initially at a ratio of 2:1 for 24 h, then at a ratio of 1:2 for the following 24 h.
To remove the remaining chloroform and ethanol, the scaffolds were placed in laboratory grade glass (600-ml PYREX™ Griffin Beakers; Thermo Fisher Scientific, Inc., Waltham, MA, USA), then deionized water (400 ml) was added, and the glass was placed on a compact digital mini rotator (#88880026; Thermo Fisher Scientific, Inc.), 37°C, with a gentle shaking speed of 50 rpm for 12 h. Subsequently, the bone fragments were rinsed in fresh deionized water for 2 h.
Deproteinization of bone
On the next stage, the bone fragments were placed in the stirrer (to remove the protein that was found in the bone and for the inactivation of prions, which causes cattle spongiform encephalopathy) and at 120 rpm the bone fragments were rinsed with 4% sodium hypochlorite for 24 hours.
To remove the remaining solvents from the deproteinized bone, the deionized water was added and stirred at 120 rpm for 72 hours, which also cleansed from sodium hypochlorite. Over the 1st 12 hours, the deionized water has been changed every 2 hours and afterwards the deionized water was changed every 12 hours.
Afterwards, bone fragments were processed with 5% hydrogen peroxide for 6 hours, which removed non-collagen protein molecules and, at the same time, deteriorated such substances as pigments, remaining lipids, toughly dissolving salts etc.
After this stage, the bone fragments were rinsed in deionized water for 10 hours and the deionized water was changed every 2 hours.
Lyophilization protocol
The process of lyophilization consisted of two stages: deep freezing of objects and thawing in a vacuum. After the seeding, the characterization of decellularized bovine bone graft was freeze-dried with a lyophilizer (Heto PowerDry PL6000 Freeze Dryer; Sjia Lab, Shenzhen, China). The temperature of the lyophilizer was set at −40°C, and the vacuum was controlled under 10–15 Pa. The thawing procedure lasted for 18–24 h.
The chamber was warmed up to 15–20°C at a rate of 0.2°C/min and sustained for 6–8 h. Following lyophilization, the decellularized bovine bone grafts were packed in disposable plastic bags (Wipak Medical, Bomlitz, Germany), following which they were sterilized with gamma-ray doses of 15 kGy and stored in sterile conditions at room temperature until use.
DNA quantification of decellularized bovine bone graft
DNA was isolated from the decellularized bovine bone graft tissue in accordance with manufacturer's protocol using a commercial extraction kit (G-spin Total DNA Extraction Mini kit; iNtRON Biotechnology, Inc., Seongnam, South Korea). The total DNA was determined using a spectrophotometer (NanoDrop 1000; Thermo Fisher Scientific, Inc.) at 260 nm.
Histology study
Samples of bovine bone were harvested prior to and following decellularization, and were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin, and Masson's trichrome staining.
Scanning electron microscopy of decellularized bovine bone graft
The decellularized bovine bone graft was dehydrated by processing with an ethanol solution prior to drying with a tousimis Samdri-780 Critical Point Dryer (Tousimis Research Corporation, Rockville, MD, USA). Following drying, all tissues were sputter coated lightly with gold and imaged on a Hitachi Scanning Electron microscope (Hitachi, Ltd., Tokyo, Japan).
Characterization of decellularized bovine bone graft
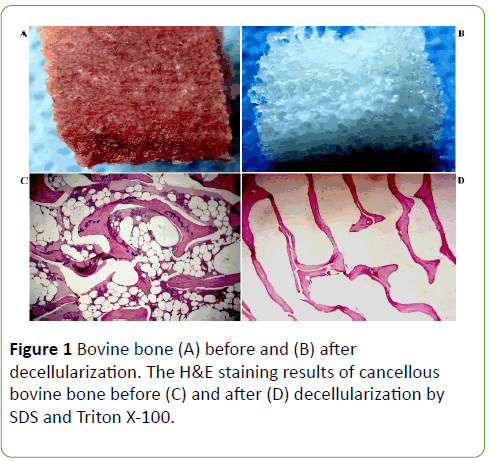
Before decellularization, since the bone fragment was containing blood cells and bone cellular components it was red color (Figures 1A and 1B). After decelluarization, the bone fragment became white which is supposed to be the natural color of bone extracellular matrix.
Furthermore, H&E staining demonstrated no presence of the bone key cellular components such as osteoblasts, osteocytes, the hematopoietic elements and adipocytes in the decellularized bone fragments (Figures 1C and 1D). Following the decellularization procedure, the residual DNA content was <1.4%.
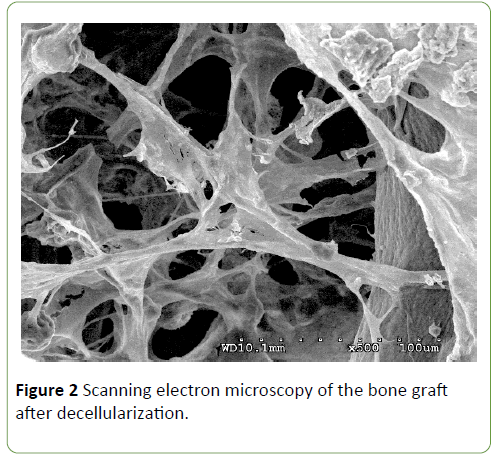
Low magnification scanning electron micrographs demonstrated decellularized bovine bone graft, observed as a mesh of collagen fibers that were intact and which were similar to the structure of natural bone. Scanning electron microscopy has also shown that the decellularized bone graft chemically and structurally is compatible with the mineralized human bone (Figure 2).
Case Presentation
We present the case of a 54-year-old female patient who in 2010 years had to car accident and suffered a major crush injury to her head resulting in a zygomatic bone defect. Autologous parietal bone graft was used for reconstruction.
However, three years after reconstruction clinical and radiographic examination showed resorbtion of autologous bone graft. The patient underwent secondary grafting with a decellularized bovine bone graft.
The patient signed a written informed consent prior to participation. The study protocol was generated according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the S. Khechinashvili University Clinic, Tbilisi, Georgia. Alginate impression material fit the patient's face and a model with the type III dental stone (COECAL™ Type III Dental Stone - GC America, Inc.) was made.
Zygomatic bone defect prosthesis was sculpted in wax (Tenastyle modelling wax, Kemdent® Associated Dental Products Ltd, Wiltshire, UK) on the cast. After completion of the wax pattern, it was evaluated to improve contour, surface texture and aesthetics of the patient’s face.
Before removal of the residual and resorbed old graft, decellularized bovine bone graft was dipped into the patient’s warm blood for several minutes prior to implantation in order to let it absorb as much blood as possible; this widely used surgical practice is based on the fact that the blood starts coagulating inside the graft and thus several growth factors and biochemical signaling molecules are released, finally enhancing and speeding up graft integration once placed into the host’s target site.
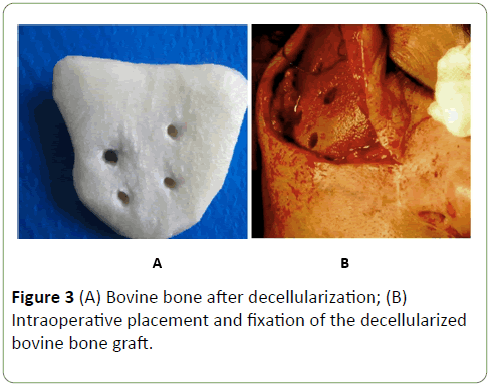
After, the insertion of the decellularized bovine bone graft into the planned position, and its fixation to the residual zygomatic bone with titanium plate, screws and wire (Figures 3A and 3B). Wound was closed layer after layer using 4-0 vicryl and 5-0 ethilon sutures.
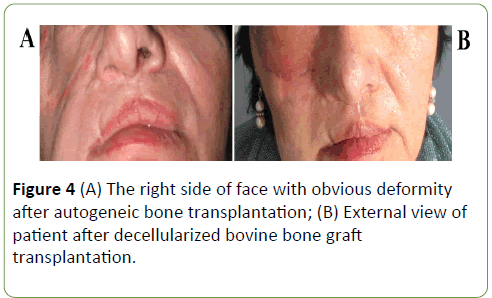
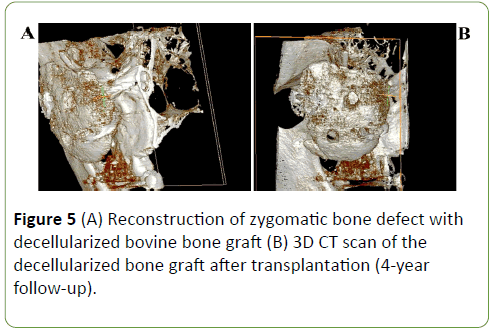
The postoperative course was uneventful, and the patient was discharged 8 days after the operation. The follow-ups at 1 month, 6 months and 1 year revealed no complications and patient was satisfied with his facial esthetic results (Figures 4A and 4B). After 4-year follow-up examination, face symmetry was recovered, and radiographic study has shown the good projection (Figures 5A and 5B) of the reconstructed site and the symmetry between the two zygomas.
Discussion
The maxillofacial bones, including zygoma, play an important role in facial contour since the shape of the face is influenced largely by the underlying osseous structure [13]. These kinds of traumatic defects cause not only esthetic but psychological disorders as well. A clear example of this was our patient, when the autologous bone graft has been resorpted throughout three years after transplantation and the patient redeveloped facial asymmetry. We think that resorption of autologous bone graft occured because the bone graft was of large size and non-vascularized,and also the host-site was weakly vasculairzed and fixation between the transplanted graft and host bone was imperfect. Of course, at reoperation the vascularized autologous bone graft would be an attractive reconstructive option, however, the patient refused this procedure.
Many articles report that the vascularized autogenous bone is a gold standard for bone defect reconstruction [3]. However, another authors think that the vascularized bone graft is not as predictable as they would like to [14,15], and indication is restricted by the high operative burden for the patient and by the availability of alternative rehabilitation methods [16].
In recent years, using the methods of decellularization has enabled to obtain the natural,biocompatible threedimensional xenograft for the reconstruction of bone defects. Removing DNA from the bone matrix is critical because this DNA may stimulate immune reactions by activating cytokine production and B-cell immunoglobulin secretion after transplantation [17,18]. Decellularized bone contains collagens type I, IV and X, and non-collagenous proteins, as well as growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor-beta 1, 2, and 3 endows it with osteoinductive properties [19,20].
There are various protocols of bone decellularization [21,22]. As it has been described by us earlier [12], following the decellularization, cancellous bone fragments acquired from bovine femur bones have a desired three-dimensional porous structure. The mesh of collagen fibers had retained their structure and was similar to natural bone. The preclinical studies that have been conducted by us have confirmed the capability of decellularized bovine bone grafts integrate with the host bone.
The most remarkable advantage of xenograft is that there is a possibility an unlimited amount of material that can be obtained from bovine bones. Bovine cancellous bones structure and morphology are similar to human cancellous bone, and additionally it has osteoconductivity ability [23]. However, the major disadvantage of xenogenous bone grafts is that it has immune response that is also stronger than that of allografts. In order to use bovine cancellous bone as bone grafts, decellularization is the first and most important subject that needs more attention [24].
According to multiple paper data, our preclinical studies on animal and this clinical case we can state that the decellularized bovine bone graft can be used for large bone defect reconstruction. This decellularized bone material retained its shape and structure and integrated with the host's bone after transplantation. However, more animal and clinical studies are required to further assess the osteogenic activity and implementation of decellularized bone grafts in clinical practice.
Many authors report on the successful clinical use of decellularized bovine bone to reconstruct bone defects [25-28]. However, the number of articles dealing with the use of declerulated bovine bone grafts of large sizes is very limited.
This clinical case indicated that the large sizes decellularized bovine bone graft retain shape and structure and integration with the host's bone within four years after transplantation. However, more animal and clinical studies are required to further assess the osteogenic activity and implementation of decellularized bone grafts in clinical practice.
Conclusion
For the large maxillofacial bone reconstruction procedures, the decellularized bovine bone graft has proven to be effective, easy to handle and biocompatible, providing excellent final aesthetic and functional results. However, more animal and clinical studies are required to further assess the osteogenic activity and integration of decellularized bone grafts with the native bone.
References
- Brydone AS, Meek D, Maclaine S (2010) Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H 224: 1329-1343.
- Rogers GF, Greene AK (2012) Autogenous bone graft: Basic science and clinical implications. J Craniofac Surg 23: 323-327.
- Springfield DS (1992) Autogenous bone grafts: Nonvascular and vascular. Orthopedics 15: 1237-1241.
- Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV (2011) Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 42: S3-15.
- Lee SH, Yoo CJ, Lee U, Park CW, Lee SG, et al. (2014) Resorption of autogenous bone graft in cranioplasty: Resorption and reintegration failure. Korean J Neurotrauma 10: 10-14.
- Friesenbichler J, Maurer-Ertl W, Sadoghi P, Pirker-Fruehauf U, Bodo K, et al. (2014) Adverse reactions of artificial bone graft substitutes: Lessons learned from using tricalcium phosphate geneXR. Clin Orthop Relat Res 472: 976-682.
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: Current concepts and future directions. BMC Med 9: 66.
- Oryan A, Alidadi S, Moshiri A, Maffulli N (2014) Bone regenerative medicine: Classic options, novel strategies, and future directions. J Orthop Surg Res 9: 18.
- Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: Recent advances and challenges. Crit Rev Biomed Eng 40: 363-408.
- Oryan A, Alidadi S, Moshiri A, Maffulli N (2014) Bone regenerative medicine: Classic options, novel strategies and future directions. J Orthop Surg Res 9: 18.
- Moshiri A, Oryan A (2012) Role of tissue engineering in tendon reconstructive surgery and regenerative medicine: Current concepts, approaches and concerns. Hard Tissue 1: 11.
- Kakabadze A, Mardaleishvili K, Loladze G, Karalashvili L, Chutkerashvili G, et al. (2017) Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze‑dried bone marrow stem cell paracrine factors. Oncology Letters 13: 1811-1818.
- Kumar SRR, Raju KV, Sunanda K (2010) Stabilization of the isolated zygomatic arch fracture using Foley's Balloon catheter. J Maxillofac Oral Surg 9: 407-409.
- Heitmann C, Erdmann D, Levin LS (2002) Treatment of segmental defects of the humerus with an osteoseptocutaneous fibular transplant. J Bone Joint Surg Am 84-A: 2216-2223.
- Wood MB, Bishop AT (2007) Massive bone defects of the upper limb: reconstruction by vascularized bone transfer. Hand Clin 23: 49-56.
- Binger T, Hell B (1999) Resorption of microsurgically vascularized bone grafts after augmentation of the mandible. J Craniomaxillofac Surg 27: 82-85.
- Pisetsky DS (1997) DNA and the immune system. Ann Intern Med 126: 169-171.
- Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786-801.
- Buckwalter JA, Glimcher MJ, Cooper RR, Recker R (1995) Bone Biology Part I: Structure, blood supply, cells, matrix and mineralization. J Bone Joint Surg Am 77: 1256-1272.
- Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO (2012) Demineralized bone matrix in bone repair: History and use. Adv Drug Deliv Rev 64: 1063-1077.
- Gardin C, Ricci S, Ferroni L, Guazzo R, Sbricoli L, et al. (2015) Decellularization and Delipidation Protocols of Bovine Bone and Pericardium for Bone Grafting and Guided Bone Regeneration Procedures. PLoS One 10: e0132344.
- Lee DJ, Diachina S, Lee YT, Zhao L, Zou R, et al. (2016) Decellularized bone matrix grafts for calvaria regeneration. J Tissue Eng 7.
- Kim SH, Shin JW, Park SA, Kim YK, Park MS, et al. (2004) Chemical, structural properties, and osteoconductive effectiveness of bone block derived from porcine cancellous bone. Journal of Biomedical Materials Research Part B: Applied Biomaterials 68: 69-74.
- Quan TM, Vu DN, Ngoc My NT, Bao Ha TL (2014) Decellularization of xenogenic bone grafts for potential use as tissue engineering scaffolds. Int J Life Sci Med Res 4: 38-45.
- Cheng CW, Solorio LD, Alsberg E (2014) Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv 32: 462-484.
- Stavropoulos A, Karring T (2010) Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J Clin Periodontol 37: 200-210.
- Cosyn J, Cleymaet R, Hanselaer L, De Bruyn H (2012) Regenerative periodontal therapy of infrabony defects using minimally invasive surgery and a collagen-enriched bovine-derived xenograft: A 1-year prospective study on clinical and aesthetic outcome. J Clin Periodontol 39: 979-986.
- Oortgiesen DA, Plachokova AS, Geenen C, Meijer GJ, Walboomers XF, et al. (2012) Alkaline phosphatase immobilization onto Bio-Gide® and Bio-Oss® for periodontal and bone regeneration. J Clin Periodontol 39: 546-555.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences