Anesthesia for Thoracic Aortic Aneurysm without Cardiopulmonary Bypass in Children
Zhang Y, Chen L, Ji H, Yang L and Yan F
Zhang Y, Chen L, Ji H, Yang L and Yan F*
Department of Anesthesiology, State Key Laboratory of Cardiovascular Diseases, National Center for Cardiovascular Diseases, Fuwai Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, P.R. China
- *Corresponding Author:
- Yan F
Department of Anesthesiology, State Key Laboratory of Cardiovascular Diseases, National Center for Cardiovascular Diseases
Fuwai Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, 100037, Beijing, P.R. China
Tel: +86 10 6513 5844
E-mail: yanfuxiafw@sina.com
Received date: March 25, 2019; Accepted date: April 15, 2019; Published date: April 22, 2019
Citation: Zhang Y, Chen L, Ji H, Yang L, Yan F (2019) Anesthesia for Thoracic Aortic Aneurysm without Cardiopulmonary Bypass in Children. Med Case Rep Vol.5 No.2:92.
Abstract
Pediatric thoracic aortic aneurysms are rare but potentially life-threatening. We present the anaesthetic management of thoracic descending aortic surgery without CPB in two paediatric patients. The rare cases firstly highlight the use of endobronchial blocker enabled good exposure of the operative field and anaesthetic experiences during the thoracic descending aortic surgery without CPB in children, which require meticulous perioperative management to limit morbidity and mortality.
Keywords
Anesthesia; Thoracic aortic aneurysm; Cardiopulmonary bypass; Congenital disorders
Introduction
Pediatric thoracic aortic aneurysms are rare but potentially life-threatening. Their aetiologies vary widely and include infection, inflammation, idiopathic or congenital disorders, genetic disease, and trauma. Large aneurysms and symptomatic aneurysms are of great concern; in such cases, rupture and death have been reported frequently enough to justify early surgical therapy [1,2]. However, data regarding thoracic aortic surgery without cardiopulmonary bypass (CPB) in children are limited. We herein describe the anesthetic management of thoracic descending aortic surgery without CPB in two pediatric patients.
Case Reports
Case 1
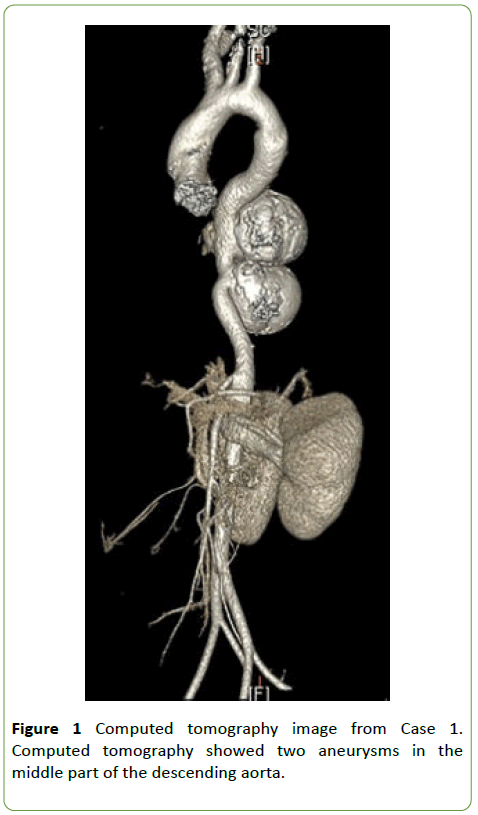
An 8-year-old boy (body weight, 23 kg) was diagnosed with a thoraco-abdominal aortic aneurysm (TAAA). Computed tomography revealed two aneurysms in the middle part of the descending aorta (maximal diameter, 41 and 52 mm, respectively) (Figure 1). Echocardiography demonstrated a left ventricular end-diastolic diameter of 36 mm and ejection fraction of 66.6% [1,2].
The TAAAs were removed and replaced with a 14-mm prosthetic graft without CPB. The operation time and descending aortic cross-clamp time were 210 and 15 min, respectively. The blood lost by the patient and 2 units of allogeneic red blood cells were pumped into a femoral artery cannula through the CPB unit to maintain adequate lower extremity arterial pressure during occlusion of the descending aorta. Acute cardiogenic pulmonary edema occurred during replacement and was controlled in a timely manner. The patient received 4 units of allogeneic red blood cells after replacement. The extubation time was 71 h. The patient stayed in the intensive care unit for 7 days and was discharged 13 days postoperatively with no complications.
Case 2
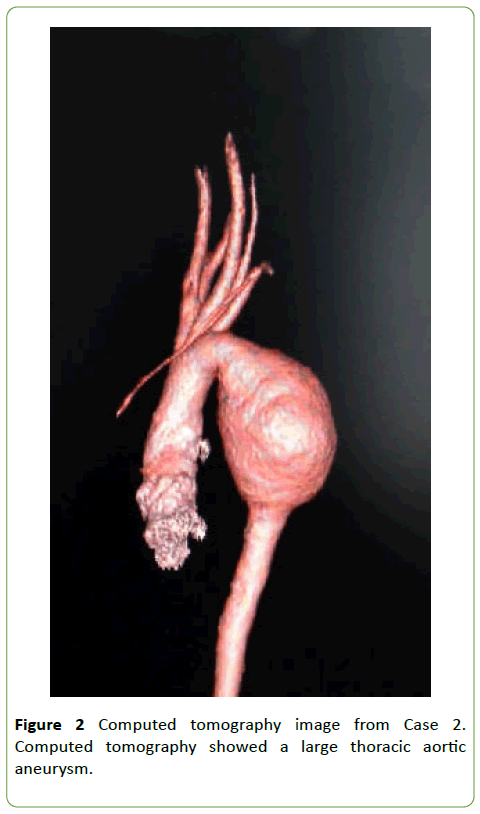
A 7-year-old girl (body weight, 20 kg) was diagnosed with a TAAA and NOTCH1 gene mutation [3]. Computed tomography revealed a large TAAA (maximal diameter, 43 mm) and depression of the pulmonary trunk and left pulmonary artery (Figure 2). Echocardiography demonstrated a left ventricular end-diastolic diameter of 37 mm and ejection fraction of 68.9%.
The TAAA was removed and replaced with a 16-mm prosthetic graft without CPB. The operation time and descending aortic cross-clamp time were 150 and 19 min, respectively. The blood lost by the patient was pumped into a femoral artery cannula through the CPB unit to maintain adequate lower extremity arterial pressure during occlusion of the descending aorta. The patient did not receive an allogeneic blood transfusion. The extubation time was 7 h. The patient stayed in the intensive care unit for 2 days and was discharged 7 days postoperatively with no complications.
Discussion
Pediatric TAAA is an uncommon disease that is highly lethal if left untreated. In patients with a TAAA, hypertension can lead to aortic dissection and/or rupture. Both children described in the current report had normal blood pressure and were premedicated with 2 mg of intravenous midazolam to decrease anxiety. We prepared atenolol and nicardipine before induction. Minimal cardiovascular responses occurred during intubation and sternal splitting because of the administration of large opioid doses.
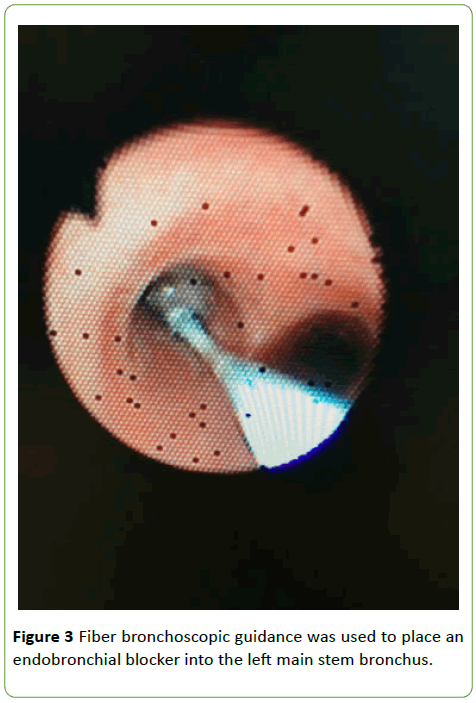
A standard tracheal tube with an inner diameter of 6.0 mm was inserted after induction. We then placed a 5-Fr endobronchial blocker into the left main stem bronchus with fibre-bronchoscopic guidance to ensure that only right-lung ventilation occurred [4] (Figure 3). Collapse of the left lung due to the endobronchial blocker enabled good exposure of the operative field from the surgical incision to the end of the replacement. Thus, the TAAA could be easily dissected and replaced. During only right-lung ventilation, we adjusted the respiratory parameters to ensure that the peripheral capillary oxygen saturation (SpO2) remained at >90% and the end-tidal carbon dioxide level remained at 35 to 45 mmHg. Upon completion of surgery, the endobronchial blocker was removed to facilitate post-operative airway management.
In Case 1, during occlusion of the descending aorta, acute cardiogenic pulmonary edema occurred in which a large amount of pink frothy sputum was discharged from the tracheal tube, the airway pressure increased from 20 cm H2O to the maximum value of 42 cm H2O, and the peripheral capillary oxygen saturation (SpO2) decreased from 98% to the minimum value of 45%. We immediately released the cuff of the endobronchial blocker and rapidly suctioned the sputum when its volume became too large to maintain an adequate airway pressure and SpO2. The patient was ventilated with 100% oxygen, and positive end-expiratory pressure of 8 cm H2O was applied to improve hypoxaemia. We intravenously administered dopamine at 3 ug/kg per min, nitroglycerine at 0.6 ug/kg per min, and furosemide at 2 mg to protect left ventricular function. After the above treatments, the pulmonary exudation was controlled, the airway pressure was maintained at 33 cm H2O, and the SpO2 was maintained at 98%. Both the blood lost by the patient and allogeneic red blood cells were pumped into the femoral artery cannula through the CPB unit during occlusion of the descending aorta, which caused left ventricular overburden and might have been the cause of the acute cardiogenic pulmonary edema. In case 2, we learned from our previous experience and only pumped the blood lost by the patient into a femoral artery cannula through the CPB unit to maintain adequate lower extremity arterial pressure during occlusion of the descending aorta. The patient recovered well.
Other necessary perioperative precautions include continuous infusion of nitroglycerine and/or intermittent intravenous nicardipine for controlled hypotension before occlusion of the descending aorta, an increase in the blood pressure, and correction of acidosis after opening of the descending aorta in accordance with the routine management of thoracic descending aortic surgery without CPB in adult patients [5].
Conclusion
Therefore, thoracic aortic surgery without CPB in pediatric patients requires meticulous perioperative management to limit morbidity and mortality. There is a need for long-term follow-up results and accumulation of further experience with anaesthesia for thoracic aortic surgery without CPB in pediatric patients.
References
- Eliason JL, Coleman DM, Criado E, Stanley JC (2016) Surgical treatment of abdominal aortic aneurysms in infancy and early childhood. J Vasc Surg 64: 1252-1261.
- Landis BJ, Ware SM, James J, Shikany AR, Martin LJ, et al. (2015) Clinical stratification of pediatric patients with idiopathic thoracic aortic aneurysm. J Pediatr 167: 131-137.
- Jiao J, Tian W, Qiu P, Norton EL, Wang MM, et al. (2018) Induced pluripotent stem cells with NOTCH1 gene mutation show impaired differentiation into smooth muscle and endothelial cells: Implications for bicuspid aortic valve-related aortopathy. J Thorac Cardiovasc Surg 156: 515-522.
- Kamra SK, Jaiswal AA, Garg AK, Mohanty MK (2017) Rigid bronchoscopic placement of fogarty catheter as a bronchial blocker for one lung isolation and ventilation in infants and children undergoing thoracic surgery: A single institution experience of 27 cases. Indian J Otolaryngol Head Neck Surg 69: 159-171.
- Vaughn SB, Le-Maire SA, Collard CD (2011) Case scenario: Anesthetic considerations for thoraco-abdominal aortic aneurysm repair. Anesthesiology 115: 1093-1102.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences