A Misdiagnosed Case Report of ò-hCG-Secreting Rectal Neuroendocrine Tumor
Shubo Wang
Department of Obstetrics and Gynecology, Women and Children’s Hospital, Weihai, China
Published Date: 2024-02-18DOI10.36648/2471-8041.10.2.362
Abstract
Background: Human Chorionic Gonadotropin (hCG), a glycoprotein hormone is widely used in clinical settings for pregnancy detection and as a sensitive marker for trophoblastic tumors. While primarily associated with placental trophoblasts, this hormone is also naturally expressed at low levels in other organs and can be elevated in nontrophoblastic malignancies. Recent studies have indicated that high β-hCG levels in nontrophoblastic tumors may signify aggressive disease and correlate with a poor prognosis.
Case presentation: This report presents a rare case involving a 44-year-old premenopausal woman misdiagnosed as ectopic pregnancy at the beginning of consultation in the hospital because of the lower abdominal pain, elevation of β-hCG levels, 90 days of amenorrhea and 54 days of vaginal bleeding. Initially, she was tested positive in a pregnancy test and was later confirmed by the presence of elevated β-hCG levels in her bloodstream. It was an atypical and rare finding in comparison to regular diagnosis of rectal cancer. Although the treatment of ectopic pregnancy was employed, the symptoms did not disappear, a systematic physical examination was required urgently and finally she was diagnosed as neuroendocrine tumor in the rectum with liver and lung metastasis through computer tomography, colonoscope and pathological examination. In addition, a biopsy of the primary rectal mass further revealed positive immunohistochemical expression of β-hCG. The chemotherapy of oxaliplatin for 4 regimens, (21 days’ interval for each regimen) was applied, and initially both of the primary and secondary tumors were reduced in size, however, one year later the tumor recured in lung, liver and rectum because of the resistance to chemotherapy. Unfortunately, the patient sacrificed as a result of brain metastasis 2 years after diagnosis.
Conclusion: It brings some lessons for gynecologists it is crucial to exclude the malignancies before diagnosis of gynecological disease for the patients with elevation of β-hCG levels. This case is distinctive due to the β-hCG-secreting rectal neuroendocrine tumor, which led to initial misdiagnosis which delayed the early treatment for rectal tumor.
Keywords
Rectal neuroendocrine tumor; Human Chorionic Gonadotropin (hCG); Lung metastasis; β-hCG elevation; Misdiagnosis
Introduction
Human Chorionic Gonadotropin (hCG) is a glycoprotein hormone secreted by the placenta after implantation of a fertilized ovum [1]. Its primary role is to support the development of the corpus luteum during early pregnancy, which in turn produces progesterone. Progesterone is crucial for maintaining the uterine lining and supporting the early stages of pregnancy. HCG is commonly used as a marker in pregnancy tests, as its presence in urine or blood indicates the potential presence of a pregnancy [2].
HCG is composed of two subunits: An alpha subunit and a beta subunit. The beta subunit is unique to hCG, and its measurement is often used in diagnostic tests, such as pregnancy tests or tests to detect certain types of cancer as a result of increased serum levels of β-hCG in some malignancies [3].
We report a case of a 44-year-old female bearing stage IV rectal neuroendocrine tumor with metastasis to the lungs and liver. Unfortunately, she was initially misdiagnosed as extrauterine pregnancy during the early stage of hospitalization as a result of elevation of hCG and left lower abdominal pain, amenorrhea,which has not been previously reported on review of literature.
Case Presentation
A Chinese female patient, 44 years old, last menstrual period was on January 10, 2020. After 40 days, there was a significant vaginal bleeding with blood clots, lasting for a week before bleeding decreased to a menstrual-like amount. Oral hemostatic drugs were taken for a week, and vaginal bleeding stopped. Increased bowel movements with urgency and heaviness, no thinning of stools. Nausea and vomiting, up to 4-5 times a day, vomiting stomach contents, occasional fever, with a maximum body temperature of 38°C.
On March 1, 2020 the patient came to our hospital for emergency treatment. Blood test showed β-hCG level of 10.27 mIU/ml. B-ultrasound shows that endometrial thickness is 10 mm. No abnormalities in the bilateral adnexal area, not hospitalized.
On March 27, 2020, Lower left abdominal pain occurred, and the patient came to our hospital for consultation. Blood β-hCG was 123 mIU/ml. B-ultrasound suggested that endometrial thickness is 14 mm, and no abnormalities were found in the bilateral adnexal area.
On 29th March, 2020, Outpatient blood test showed β-hCG: 142 mIU/ml, progesterone is 0.53 ng/ml. Diagnostic curettage was performed, and pathology suggested (uterine cavity) proliferative phase-like endometrium.
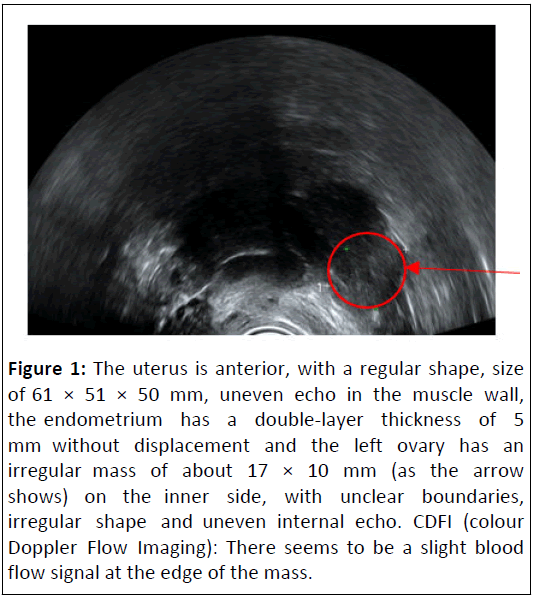
On 8th April, 2020, Blood β-hCG: 218.8 mIU/ml. the ultrasound showed the left ovary had an irregular mass of about 17 × 10 mm on the inner side, with unclear boundaries (Figure 1).
Figure 1: The uterus is anterior, with a regular shape, size of 61 × 51 × 50 mm, uneven echo in the muscle wall, the endometrium has a double-layer thickness of 5 mm without displacement and the left ovary has an irregular mass of about 17 × 10 mm (as the arrow shows) on the inner side, with unclear boundaries, irregular shape and uneven internal echo. CDFI (colour Doppler Flow Imaging): There seems to be a slight blood flow signal at the edge of the mass.
On 9th April, 2020, A single intramuscular injection of 80 mg methotrexate was administered for embryonic termination. At the same time, liver protection treatment was given, and liver function tests showed Aspartate Aminotransferase (AST) 55 U/L, Alanine Aminotransferase (ALT) 491 U/L, alkaline phosphatase 368 U/L.
On 11 April, 2020, at 22:00, The patient developed a fever with no cough, sputum, abdominal pain, or diarrhea, with mild discomfort in the chest. Rechecking showed β-hCG of 165.8 mIU/ ml. Blood routine showed a white blood cell count of 13.56 × 109/L, neutrophil ratio of 84.20%, higher than normal and hemoglobin of 67 g/L. Anti-inflammatory treatment was given.
On 19th April, 2020, The patient again had a fever with a temperature of 38.6°C, no cough, and sputum. Physical examination revealed no redness and swelling in the throat, abdominal distension, a palpable mass in the upper right abdomen with tenderness and mild tenderness on light pressure in the lower left abdomen. Gynecological examination showed no tenderness in the uterine body and bilateral adnexal areas. The patient had bowel movements more than ten times, with a small amount. Blood routine reported a white blood cell count of 21.47 × 109/L, neutrophil ratio of 84.10%, CRP 94.723 mg/L, erythrocyte sedimentation rate 46 mm/h, interleukin-6, 85.55 pg/ml and calcitonin 0.523 ng/ml. Liver function tests showed adenosine deaminase 21 U/L, AST 70 U/L, ALT 636 U/L, alkaline phosphatase 553 U/L, higher than normal. B-ultrasound showed no displacement of the endometrium on both sides, thickness of 5 mm, a cystic mass in the right adnexal area with a size of about 28 × 23 mm, transparent and a fluid-filled dark area with a range of 54 × 20 mm behind the uterus.
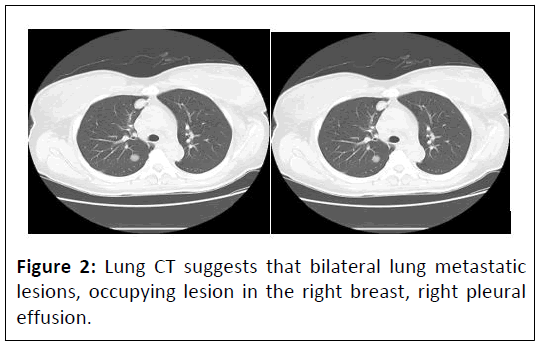
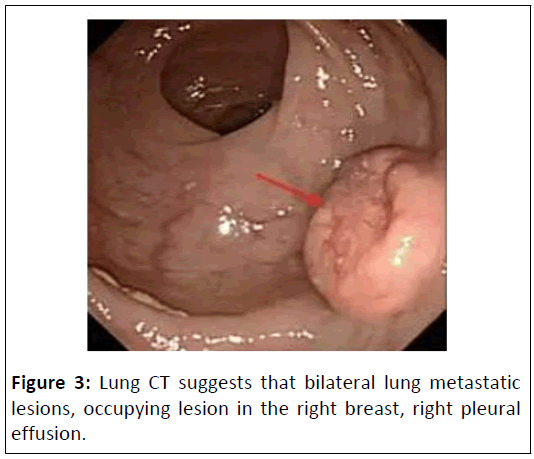
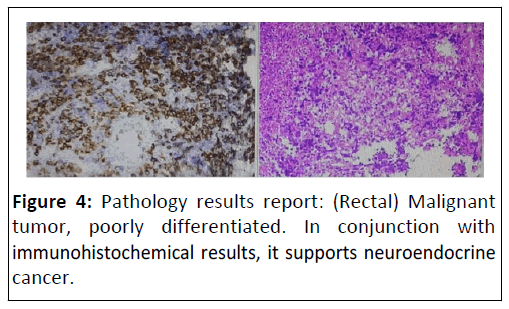
On 18th April, 2020, the CT results were reported as follows: Figure 2, Bilateral lung metastatic lesions. Occupying lesion in the right breast. Right pleural effusion. Metastatic lesions in the liver. Thickening of the colon wall in the sigmoid colon. Multiple enlarged lymph nodes around the porta hepatis, main artery and in the abdominal cavity. Fecal occult blood test positive. Aspiration of a pale yellow fluid (approximately 8 ml) from the posterior vaginal cul-de-sac, subjected to DNA quantification cytology examination, revealing a small amount of DNA tetraploidy abnormalities. Blood β-hCG level of 183.6 mIU/ml. Colonoscopy examination showed, at a distance of 9-18 cm from the anus, a huge irregular mucosal elevation affecting approximately 4/5 of the circumference of the bowel. The surface was congested and ulcerated and the biopsy revealed adenocarcinoma of the rectum, moderately differentiated. Pathology results reports: Rectal neuroendocrine carcinoma (Figure’s 3 and 4).
Immunohistochemistry results are given in Table 1. Synaptophysin (SY) (+), CD56 (focal +), Cytokeratin (CK) low (+), Chromogranin A (CgA) (+), CDX2 (-), Vilin (-), Ki67 (approximately 95% +).
| Immunohistochemistry | Results |
|---|---|
| Synaptophysin (SY) | + |
| CD56 | + |
| Cytokeratin (CK) low | + |
| Chromogranin A (CgA) | + |
| CDX2 | - |
| Vilin | - |
| Ki67 | + |
Table 1: Immunohistochemistry results.
The chemotherapy of oxaliplatin for 4 regimens, (21 days interval for each regimen) was applied and initially both of the primary and secondary tumors were reduced in size, however, one year later the tumor recured in lung, liver and rectum because of the resistance to chemotherapy. Unfortunately, the patient sacrificed as a result of brain metastasis 2 years after diagnosis.
Results and Discussion
Rectal Neuroendocrine Tumors (NETs) are a subset of neuroendocrine tumors that originate in the rectum. Neuroendocrine tumors arise from cells that have both nerve and endocrine features and they can produce hormones [4]. Rectal NETs are relatively rare compared to other types of rectal tumors, such as adenocarcinomas. The symptoms of rectal NETs can vary but may include changes in bowel habits, abdominal pain, rectal bleeding, or symptoms related to hormone production, depending on the speci ic type of NET [5].
Metastases to the liver, lungs and bone are common at presentation, Rectal Neuroendocrine Tumors (NETs) are characterized by a risk of metastatic disease ranging from 3% to 60% [6]. Most metastases occur in the liver, bones and lungs, while intracranial metastases are extremely rare [7].
Rectal carcinoid tumors are rare, making up only 1.1% to 1.3% of all rectal tumors; however, the incidence of rectal carcinoids is increasing, most likely due to the increased availability of screening endoscopy [8].
Paraneoplastic disorders are usually caused by secretion of serotonin from the tumor, which is seen more commonly in patients with larger or more invasive carcinoids [9]. Our patient had an invasive tumor with the elevation of hCG. There is no case report for rectal carcinoid tumors with increased hCG.
Human Chorionic Gonadotropin (hCG) is a hormone produced by the placenta during pregnancy. It is commonly known as the "pregnancy hormone" and is one of the earliest markers of pregnancy. In the early weeks of pregnancy, hCG plays a crucial role in maintaining the corpus luteum, which, in turn, produces progesterone [10]. Progesterone is essential for maintaining the uterine lining and supporting the early stages of pregnancy [11].
In addition to its role in pregnancy, elevated levels of hCG can be associated with certain types of tumors, including germ cell tumors and some non-seminomatous testicular cancers. Monitoring hCG levels is often part of the diagnostic and followup process in these cases [12].
The alpha subunit of hCG is structurally similar to the alpha subunits of other glycoprotein hormones such as Luteinizing Hormone (LH), Follicle-Stimulating Hormone (FSH) and Thyroid- Stimulating Hormone (TSH) [13]. The common alpha subunit provides a shared structural framework for these hormones. Unlike the alpha subunit, the beta subunit is unique to hCG. The specific sequence of the beta subunit is what distinguishes hCG from other glycoprotein hormones [14].
The beta subunit of human Chorionic Gonadotropin (hCG) is associated with various tumors and monitoring its levels can be clinically relevant for diagnostic and prognostic purposes [15]. Although hCG is mainly produced by placental syncytiotrophoblast cells, it is also produced at low levels in other organs.
Elevation of β-hCG in serum have been shown to occur in various cancers of the GI tract, mostin biliary (60%), pancreatic (46%), gastric cancer (40%), and liver (20%) and colorectal cancer (15%) [16].
Adverse prognostic factors in rectal neuroendocrine tumors encompass lymph node metastasis, tumor stage, lymphovascular or perineural invasion and the overall cancer stage. Notably, lymph node positivity stands out as one of the most significant predictors linked to decreased survival [17]. Elevated levels of β- human Chorionic Gonadotropin (β-hCG) can be associated with certain tumors and are considered a potential marker for poor prognosis in some tumors.
Yamaguchi et al. observed the presence of hCG-positive tumour cells was related to the aggressive local invasion and metatases. In addition, colorectal cancer patients with increased hCG level had a poor prognosis [18].
Lundin et al. reported 20 colorectal cancer patients with positive β-hCG on both histology and serology had a 5-year survival of 21% versus 62% in the group with no expression both in tissue or serum [19].
Our patient initially had a low level of β-hCG, however several days later β-hCG was increasing, which indicated the tumor was increasing and became more aggressive.
Surgery for Rectal Neuroendocrine Tumors (NETs) is the main effective treatment in the early and medium stage [20].
Surgical resection of metastases is recommended, as a result of a better progression free survival rate which is associated with complete resection of liver, lung and peritoneal metastases. A multidisciplinary evaluation of patients with metastatic colorectal cancer should be processed to choose the appropriate pathway [21].
Recent treatment strategies for advanced stage IV colorectal cancer involving chemotherapy have demonstrated enhanced, albeit modest, extension of survival duration.
More and more novel treatments are being developed as a result of the significant toxicity of standard chemotherapy.
Research has been exploring the potential of Active Specific Immunotherapy (ASI), with certain studies investigating the use of hCG as a target.
Moulton and colleagues conducted a phase II randomized controlled trial, which included 77 patients diagnosed with metastatic colorectal cancer. These patients were divided into two groups, each receiving different doses of a synthetic vaccine. This vaccine specifically targeted the C-terminal peptide of human Chorionic Gonadotropin (hCG). The study aimed to assess the efficacy and safety of the vaccine in treating colorectal cancer by leveraging the unique properties of the hCG peptide.
Although no significant differences were observed in overall antibody response and survival rates, the study did find a notable distinction based on anti-hCG antibody levels. Patients who developed anti-hCG antibody levels at or above the median value demonstrated a median survival of 45 weeks. In contrast, those with antibody levels below the median experienced a considerably shorter median survival of just 24 weeks. This suggests a potential correlation between higher anti-hCG antibody levels and extended survival in the patient cohort.
Conclusion
In conclusion, we report an uncommon instance of paraneoplastic β-hCG production originating from a rectal neuroendocrine tumor, a rarity in medical literature. Emerging research suggests that elevated serum β-hCG levels could serve as an independent prognostic marker, indicative of a more aggressive disease trajectory and poorer outcomes in colorectal cancer. This case highlights the potential of β-hCG as a significant biomarker in colorectal malignancies. Furthermore, investigational studies are actively exploring therapeutic interventions targeting β-hCG, paving the way for potentially groundbreaking treatments in this domain.
Availability of Data and Materials
All data sets on which the conclusions of the case report are based are to be available as a medical record document and available from the corresponding author on reasonable request from the editors.
Acknowledgements
We acknowledge the patient’s cooperation and all the teams for this patient management.
Funding
No funding.
Contributions
The author is involved in concept design, preparation, critical analysis and revision of the manuscript and is involved in the management of the patient.
Ethics Approval and Consent to Participate
The institution does not require ethical approval for the publication of a single case report.
Consent for Publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this Journal.
Competing Interests
There is no potential con lict of interest concerning the research, authorship, and/or publication of this article.
References
- Cook-Andersen H, Chuan SS, Maas K, Rosencrantz MA, Su HI, et al. (2015) Lack of Serum anti-Mullerian hormone responses after recombinant human chorionic gonadotropin stimulation in women with polycystic ovary syndrome. J Clin Endocrinol Metab 100: 251-257.
[Crossref], [Google Scholar], [Indexed]
- Devoto L, Henríquez S, Kohen P, Strauss JF (2017) The significance of estradiol metabolites in human corpus luteum physiology. Steroids 123: 50-54.
[Crossref], [Google Scholar], [Indexed]
- Stenman UH, Alfthan H, Hotakainen K (2004) Human chorionic gonadotropin in cancer. Clin Biochem 37: 549-561.
[Crossref], [Google Scholar], [Indexed]
- Yang Y, Wang XY, Duan C, Wang ZJ, Sheng HY, et al. (2023) Clinicopathological characteristics and its association with digestive system tumors of 1111 patients with Schistosomiasis japonica. Sci Rep 13: 15115.
[Crossref], [Google Scholar], [Indexed]
- Purysko AS, Coppa CP, Kalady MF, Pai RK, Leão Filho HM, et al. (2014) Benign and malignant tumors of the rectum and perirectal region. Abdom Imaging 39: 824-852.
[Crossref], [Google Scholar], [Indexed]
- Kazi M, Patkar S, Saklani A (2023) Simultaneous laparoscopic liver metastasectomy and intersphincteric resection for neuroendocrine tumor of the rectum by natural orifice specimen extraction surgery. J Minim Invasive Surg 26: 215-217.
[Crossref], [Google Scholar], [Indexed]
- Lim KHJ, Valle JW, Lamarca A (2019) Unusual skull base metastasis from neuroendocrine tumor: A case report. J Med Case Reports 13: 273.
[Crossref], [Google Scholar], [Indexed]
- Cha B, Shin J, Ko WJ, Kwon SK, Kim H (2022) Prognosis of incompletely resected small rectal neuroendocrine tumor using endoscope without additional treatment. BMC Gastroenterol 22: 293.
[Crossref], [Google Scholar], [Indexed]
- Rison RA, Shepphird JK, Beydoun SR (2016) When to write a neurology case report. J Med Case Reports 10: 1-4.
- Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, et al. (2017) hCG: Biological functions and clinical applications. Int J Mol Sci 18: 2037.
[Crossref], [Google Scholar], [Indexed]
- Czyzyk A, Podfigurna A, Genazzani AR, Meczekalski B (2017) The role of progesterone therapy in early pregnancy: From physiological role to therapeutic utility. Gynecol Endocrinol 33: 421-424.
[Crossref], [Google Scholar], [Indexed]
- Couvelard A, Paraf F, Vidaud D, Dubois S, Vidaud M, et al. (2004) Human chorionic gonadotrophin beta expression in malignant Barrett's oesophagus. Virchows Arch 445: 279-84.
[Crossref], [Google Scholar], [Indexed]
- Fares F (2006) The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: Development of agonists and antagonists. Biochim Biophys Acta 1760: 560-567.
[Crossref], [Google Scholar], [Indexed]
- Stenman UH, Alfthan H, Hotakainen K (2004) Human chorionic gonadotropin in cancer. Clin Biochem 37: 549-61.
[Crossref], [Google Scholar], [Indexed]
- Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL (2002) Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: Antibody response is associated with improved survival. Clin Cancer Res 8: 2044-2051.
[Google Scholar], [Indexed]
- Albrecht J, Meves A, Bigby M (2005) Case reports and case series from Lancet had significant impact on medical literature. J Clin Epidemiol 58: 1227–32.
[Crossref], [Google Scholar], [Indexed]
- Sohn B, Kwon Y, Ryoo SB, Song I, Kwon YH, et al. (2017) Predictive factors for lymph node metastasis and prognostic factors for survival in rectal neuroendocrine tumors. J Gastrointest Surg 21: 2066-2074.
[Crossref], [Google Scholar], [Indexed]
- Yamaguchi A, Ishida T, Nishimura G, Kumaki T, Katoh M, et al. (1989) Human chorionic gonadotropin in colorectal cancer and its relationship to prognosis. Br J Cancer 60: 382-384.
[Crossref], [Google Scholar], [Indexed]
- Lundin M, Nordling S, Lundin J, Alfthan H, Stenman UH, et al. (2001) Tissue expression of human chorionic gonadotropin beta predicts outcome in colorectal cancer: A comparison with serum expression. Int J Cancer 95: 18-22.
[Crossref], [Google Scholar], [Indexed]
- Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, et al. (2021) Diagnosis and management of rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel) 11: 771.
[Crossref], [Google Scholar], [Indexed]
- Hernandez DO, Yilmaz S, Steele SR (2023) Stage IV colorectal cancer management and treatment. J Clin Med 12: 2072.
[Crossref], [Google Scholar], [Indexed]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences