22q11.2 Deletion Syndrome: Copy Number Variation Analysis in Two Patients
Dong Wu#, Weili Shi#, Hui Zhang, Yan Chu and Shixiu Liao
Dong Wu#, Weili Shi#, Hui Zhang, Yan Chu and Shixiu Liao*
Medical and Genetics Institute of Henan Province, Henan Provincial People’s Hospital, Zhengzhou University, Zhengzhou, PR China
- *Corresponding Author:
- Shixiu Liao
Medical and Genetics Institute of Henan Province, Henan Provincial People’s Hospital, Zhengzhou University, Zhengzhou, PR China.
Tel: +86-371-87160116
E-mail: ychsliao@126.com
Received date: January 28, 2016; Accepted date: May 13, 2016; Published date: May 16, 2016
Citation: Wu D, Shi W, Zhang H, et al. 22q11.2 Deletion Syndrome: Copy Number Variation Analysis in Two Patients. Med Case Rep. 2016, 2:2.
Abstract
Background: 22q11.2 deletion syndrome is the most common microdeletion syndrome. It is caused by an autosomal dominant microdeletion of chromosome 22 at the long arm 11.2 band. It is difficult to diagnose of this syndrome because of its multiple phenotypes.
Methods and findings: In this study, we identified two cases of 22q11.2 deletion syndrome by array based comparative genomic hybridization (aCGH). These two cases exhibited atypical anomaly examined by aCGH and they exhibited different presentations although aCGH showed the same deletion.
Conclusion: 22q11.2 deletion syndrome is a difficult diagnosed disease which could be efficiently detected by aCGH. The patients could exhibit different phenotypes even when aCGH showed the same deletion. These findings will provide useful information to better understand 22q11.2 deletion syndrome.
Keywords
22q11.2 deletion syndrome; Copy number variation; Array based comparative genomic hybridization (aCGH)
Introduction
22q11.2 chromosomal region contains several paralogous low copy repeats (LCRs), which predisposes to de novo genomic rearrangement at this locus. Hemizygous deletions of this region, also known as copy number variations (CNVs), results in 22q11.2 deletion syndrome (22q11.2 DS). The 22q11.2 DS affects about 1 in 4000 live births. Patients of 22q11.2 DS have high prevalence of intellectual disability and psychiatric disorders [1] and death related to this syndrome has also been reported. The 22q11.2 DS is much variable with more than 180 features. It showed wide spectrum of clinical presentations ranging from mild to severe, which led to difficult diagnosis of this disease. In most cases, 22q11.2 DS is caused by a hemizygous deletion of 3 million base pairs of DNA that encompasses approximately 40 known genes. Smaller nested deletions of 1.5 million base pairs that span 34 known genes also were reported [2,3].
In this study, we reported two cases of 22q11.2 DS showed atypical anomaly of 2.5 M deletion identified by array based comparative genomic hybridization (aCGH). And these two cases showed different presentations although aCGH revealed the same deletions.
Case Description
The patient 1 shows atypical characteristic features for 22q11.2 DS. He is an one-year old boy who exhibits intellectual disabilities, developmental delay, dyskinesia, patent foramen ovale, laryngeal cartilage dysplasia, varus, gastric torsion, cerebral palsy and hearing loss. After peripheral blood was collected from this patient, both Karyotyping analysis and aCGH analysis were performed, while karyotyping analysis showed negative result.
The second sample was from a pregnant woman at 20 weeks of gestation. Fetal ultrasound indicated abnormality of eyes structures and ascites. The pregnant chose definitive prenatal diagnosis through amniocentesis after received genetic counseling. Karyotyping analysis of amniotic fluid did not detect any abnormal. Afterwards, aCGH was carried out with amniotic fluid.
Briefly, genomic DNA was extracted from peripheral blood or amniotic fluid with TIANamp Blood DNA Kit (TIANGEN, China) according to manufacturer’s instructions. aCGH was performed using Agilent SurePrint G3 Human Genome Microarray 4 × 180K (Agilent Technologies, USA). Labeling and hybridization were performed via Agilent Genomic DNA Enzymatic Labeling Kit (Agilent Technologies, USA). Then array slides images were acquired on an Agilent scanner. Then the data were processed with Feature Extraction software (v10.7). Results were further analyzed by Agilent Genomic Workbench (v6.5) and Agilent Cytogenomics (v2.7.7.0) based on Human Genome build 19, and further interpreted by databases consultation.
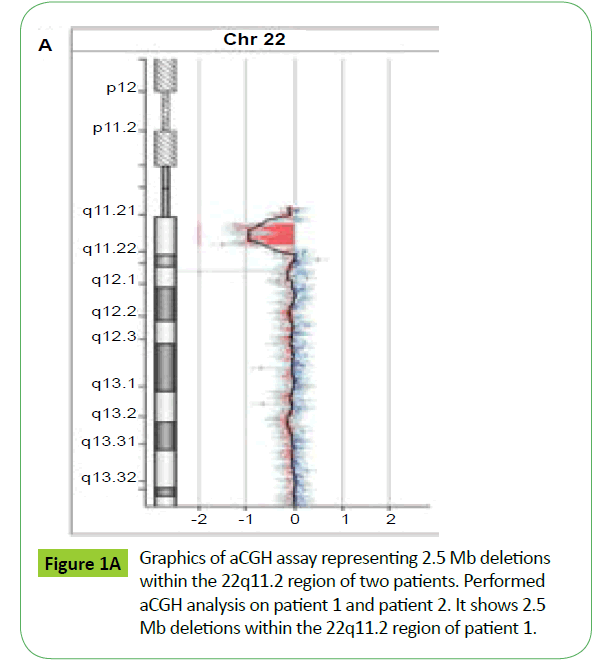
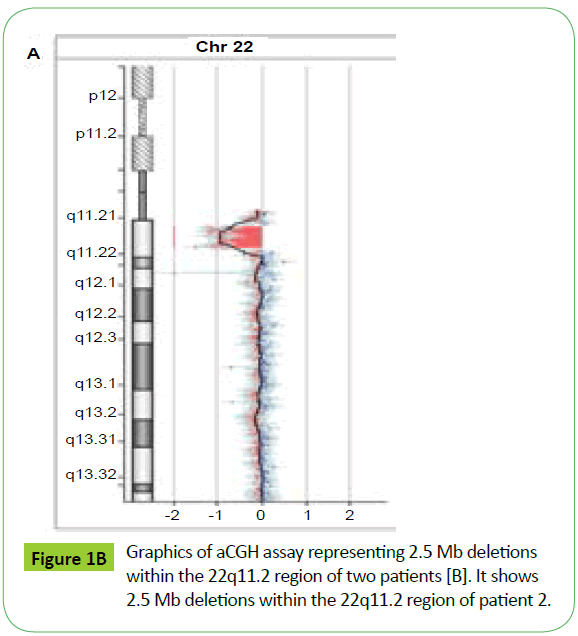
aCGH analysis showed that patient one covered about 83.3% deletion of the typically deleted region (TDR) of 22q11.2 deletion syndrome (Figures 1A and 1B). The fetal also covered about 83.3% of the TDR (Figure 1), which is the same as the patient one showed by aCGH.
Comments
aCGH is a powerful tool to detect 22q11.2 deletion syndrome. Different patients could show same deletion examined by aCGH even when they exhibited different presentations.
Ethical Consideration
Ethical approval was obtained from the Ethics Committee of Henan Provincial People’s Hospital, China. The patients involved in this study signed the consent documents.
Discussion
It is known that there are 4 LCRs in proximal region and 4 another smaller LCRs in distal region in 22q11 chromosome based on their chromosome order. Different size and different location of deletions usually exhibited different phenotypes. In this study, the two patients showed different clinical presentations while they shared the same deletion detected by aCGH. Since 22q11.2 DS is a disease that has different phenotypes among different patients, it is difficult in diagnosis. Some mild and atypical phenotypes may not be diagnosed in time and correctly in this disease. Therefore, array based comparative genomic hybridization is a powerful technology that used to identify 22q11.2 DS.
So far, the mechanism of 22q11.2 deletion syndrome remains largely elusive. It was suggested that LCRs mediated rearrangements of 22q11.2 [4]. As how LCRs mediated deletions in 22q11.2 need to be further explored. 4 proximal LCRs show a complex modular structure, and the modular share high level of sequence identity. This may lead to misalignment during meiosis and unequal interchromosomal recombination. So far, LCR mediated non-allelic homologous recombination (NAHR) in genomic rearrangement has already been widely accepted [5]. Afterwards, it is reported that 4 distal LCRs also were involved in this deletion syndrome [6].Further studies would provide more information that helps to better understand the mechanism of 22q11.2 DS.
Conclusion
In conclusion, 22q11.2 DS is a variable disease exhibited different phenotypes even when aCGH showed the same deletions. aCGH is a pangenemic screening technology with higher resolution and rapid breakpoint localization compared to traditional methods in genomic disorders. It is a good option when the phenotype is atypical and not a clear diagnosis is obtained in clinical in 22q11.2 DS, and even other deletion syndromes.
References
- Drew L, Crabtree G,Markx S, Stark L, Chaverneff F, et al. (2011) The 22q11.2micro deletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International Journal of Developmental Neuroscience 29: 259-281.
- Edelmann L, Pandita R, Morrow E (1999) Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. American Journal of Human Genetics64: 1076-1086.
- Shaikh T, Kurahashi H, Saitta S, O'Hare, Hu P, et al. (2000) Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Human Molecular Genetics 9: 489-501.
- Tutulan A, Budisteanu M, Papus M, Dupont M, Blancho D, et al. (2012)Atypical presentations of 22q11.2 deletion syndrome: explaining the genetic defects and genome architecture. Psychiatry Research 197: 356-357.
- Pires R, PiresL, Vaz S, Maciel P, Anjos R, et al. (2014) Screening of copy number variants in the 22q11.2 region of congenital heart disease patients from the Sao Miguel Island,Azores, revealed the second patient with a triplication. BMC Genetics 7:115.
- Shaikh T, Connor J, Pierpont M, McGrath J, Hacker A, et al. (2007) Low copy repeats mediate distal chromosome 22q11.2 deletions: sequence analysis predicts breakpoint mechanisms. Genome Research 17: 482-491.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences