Undifferentiated Embryonal Sarcoma of Liver- A Rare Case Report
Nisha Khanna, Shailee Mehta, Alok Ranjan, Biren Parikh, Dhaval Jetly and Vaibhavi H Dhimmar
DOI10.21767/2471-8041.S1-011
Nisha Khanna1*, Shailee Mehta1, Alok Ranjan1, Biren Parikh1, Dhaval Jetly1 and Vaibhavi H Dhimmar2
1Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India
2Department of Pathology, BJ Medical College, Ahmedabad, Gujarat, India
- *Corresponding Author:
- Nisha Khanna
Department of Pathology, Gujarat Cancer Research Institute
Civil Hospital Campus, Asarwa, Ahmedabad, Gujara, India
Tel: 079 2268 8000
Fax: +58212276.12583
E-mail: Umircaracas@yahoo.es
Received Date: Janurary 23, 2018; Accepted Date: January 29, 2017; Published Date: January 31, 2017
Citation: Khanna N, Mehta S, Rajan A, Parikh B, Jetly D, et al. (2018) Undifferentiated Embryonal Sarcoma of Liver- A Rare Case Report. Med Case Rep Vol.4 No. S1:011. DOI: 10.21767/2471-8041.S1-011
Abstract
Undifferentiated embryonal sarcoma of liver (USEL) is a rare primary hepatic malignancy principally affecting patients of pediatric age group. We present a case undifferentiated embryonal sarcoma of liver in a 1-year old female. She presented with complaints of abdominal distension and vomiting for 2 weeks. Ultrasonography showed grossly enlarged liver with multiple heterogenous lesions. Computed tomography revealed presence of large exophytic lesion of size 11.3 × 7 cm involving left lobe of liver. Ultrasound guided biopsy was performed, and specimen was sent for histopathological examination. On Microscopic examination undifferentiated round to spindle cells in myxoid background were seen. Immunohistochemistry revealed positivity for vimentin and AE1 thus proving undifferentiated embryonal sarcoma of liver. Diagnosis is often challenging because of overlapping epidemiological, clinical, radiological findings with those of other liver tumors.
Keywords
Undifferentiated sarcoma; Liver; Tumors
Introduction
Undifferentiated embryonal sarcoma of liver (USEL) is a rare primary hepatic malignancy principally affecting patients of pediatric age group. We present a case undifferentiated embryonal sarcoma of liver in a 1-year old female. She presented with complaints of abdominal distension and vomiting for 2 weeks. Ultrasonography showed grossly enlarged liver with multiple heterogenous lesions. Computed tomography revealed presence of large exophytic lesion of size 11.3 × 7 cm involving left lobe of liver. Ultrasound guided biopsy was performed, and specimen was sent for histopathological examination. On Microscopic examination undifferentiated round to spindle cells in myxoid background were seen. Immunohistochemistry revealed positivity for vimentin and AE1 thus proving undifferentiated embryonal sarcoma of liver. Diagnosis is often challenging because of overlapping epidemiological, clinical, radiological findings with those of other liver tumors.
Case Presentation
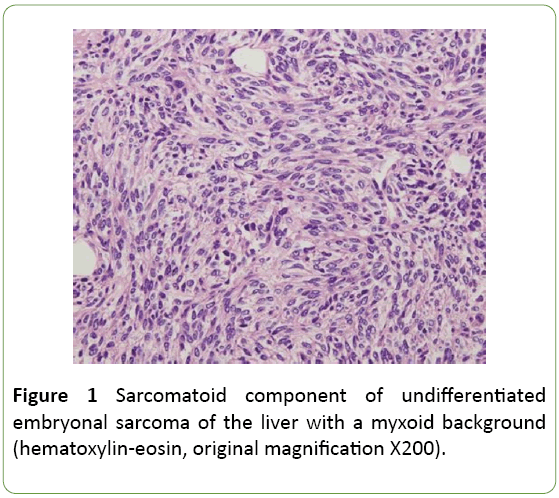
A 1-year-old female child presented to our hospital with chief complaints of abdominal distension, vomiting since 2 weeks and respiratory distress since 1 week. Physical examination revealed pale conjunctiva and skin and enlargement of liver without percussion pain. Her hemogram revealed hemoglobin 6.6 g/dl, total cont 4000/cumm and platelets 1,36,000/cumm. Liver functional test showed that her alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, direct bilirubin, and albumin were 81 U/L, 129U/L, 241 U/L, 1.4 mg/dl, 0.83 mg/dl and 3.51 g/dl respectively. Series hepatitis markers (hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus, hepatitis E virus and Epstein-Barr virus) were negative. α-fetoprotein, carcinoembryonic antigen and CA19-9 concentrations were also normal. Human immunodeficiency virus and syphilis antibodies were negative. Coagulation profile was normal (Figure 1).
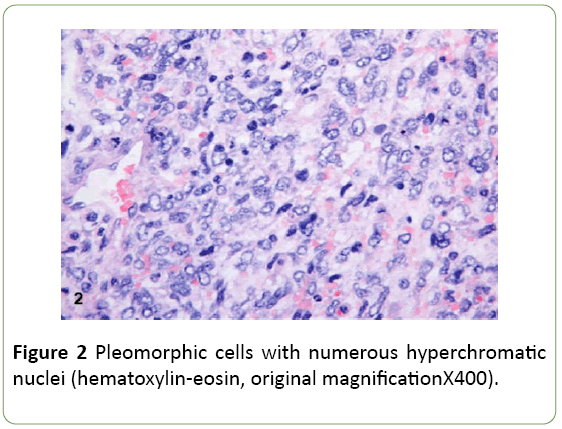
Abdominal ultrasonography showed a grossly enlarged liver (20 cm) with presence of multiple heterogenous echotexture lesions in both lobes of liver largest measuring 14 × 11 cm in right lobe of liver. CT scan abdomen revealed presence of large lobulated heterogeneously enhancing lesion abutting anterior abdominal wall and displacement of multiple small bowel loops, transverse colon and part of pancreatic head. Another hypodense lesion adjacent to caudate lobe of liver at porta was present encasing portal vein, splenic artery, hepatic artery and origin of celiac trunk. Lesion abuts IVC, right adrenal gland, right kidney. Multiple nodular lesions were found in bilateral lower lobes of lungs indicating metastasis to lungs (Figure 2).
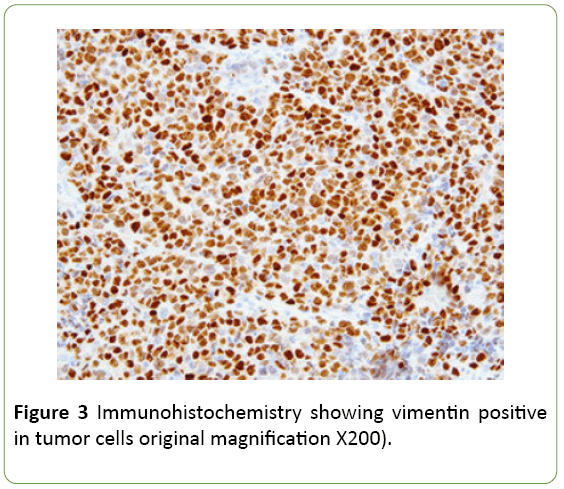
Ultrasound guided biopsy was performed, and specimen was sent to histopathological examination. On gross examination specimen consisted of multiple linear grey white soft to firm tissue pieces measuring about 0.5 to 1 cm. Microscopic examination shows undifferentiated type round to spindle cells in myxoid background. Tumor cells had eosinophilic cytoplasm with hyperchromatic nucleus and small nucleoli. Entrapped bile ducts were present. Possibility of malignant mesenchymoma was suggested. On immunohistochemistry vimentin, AE1, and CK7 were found to be positive. S100 was focally positive. Desmin, PLAP, AFP were negative thus confirming undifferentiated embryonal sarcoma of liver (Figure 3).
Discussion
UESL is a rare hepatic mesenchymal tumor that was first reported and classified by Stocker et al. in 1978 [1-3]. Undifferentiated embryonal sarcoma of the liver is often considered a malignant evolution of Mesenchymal Hamartoma. The incidence of the disease has no significant gender difference. In addition, 90% of patients are children aged 610 years and the disease accounts for 5-8% of hepatic tumors in children [1]. The tumor is mainly localized or found in the hepatic right lobe (59%), while it rarely develops in the hepatic left lobe (22%) or the bilateral lobe (20%). UESL typically has a diameter of 10-25 cm with a solitary clear boundary. Hemorrhage, necrosis and cystic degeneration are frequently observed while clinical manifestations include abdominal mass, pain, fever and rarely jaundice [4,5].
An ultrasonography scan reveals a mixed echoic mass containing an irregular anechoic region or multiple small capsular spaces of different sizes. The solid region exhibits a mixture of high- and low-level echoes. By contrast a computed tomography (CT) scan reveals cystic lesions with low density that is reflected as a fluid. Therefore, the difference between CT and ultrasonography results is a critical characteristic of this disease. However, both scans reveal cystic lesions when hemorrhage, necrosis and liquefaction account for the major part of the tumor [6]. An enhanced CT scan of the tumor solid part, septation and pseudo-capsule shows different degrees of reinforcement. However, in the present study, MRI revealed that the T1W1 scan mainly detected a low or equal signal and high signal foci, which were reflection of a hemorrhage in the tumor. Additionally, the T2W1 scan frequently exhibited a mixed high signal. Positron emission tomography (PET)-CT scans are also used in the diagnosis of UESL. They have a critical diagnostic value for UESL patients, particularly those with metastasis of an extra-hepatic organ.
Undifferentiated embryonal sarcoma of the liver usually occurs as a single and well-circumscribed lesion grossly. The well-demarcated appearance is created by a fibrous pseudocapsule [4], which is formed by compressed liver parenchyma. Cut surface reveals a heterogeneous appearance of grey-white, glistening solid tumor alternating with cystic, gelatinous areas. In addition, dark-brown areas of hemorrhage and yellow, softer areas of necrosis are often seen grossly. Microscopically, the pseudocapsule separates the lesion from the surrounding hepatic parenchyma. Cords and clusters of hepatocytes are commonly seen within the pseudocapsule and at the peripheral margin of the lesion. The solid component of UESL appears sarcomatoid with a myxoid background. The cells are spindle or stellate shaped with inconspicuous nucleoli and ill-defined cell borders. Multinucleated cells and bizarre cells with hyperchromatic nuclei are often seen between the sarcomatoid cells [2]. Numerous mitotic figures are easily identified throughout the tumor. Characteristically, eosinophilic globules can be seen in the tumor cell cytoplasm and extracellular matrix. These globules are positive for periodic acid–Schiff and resistant to diastase digestion. Many studies have shown that UESL does not have a specific immunophenotype. The variable expression of histiocytic, muscle, and epithelial markers is suggestive of primitive stem cells as the origin of this lesion. Most cases are positive for vimentin, desmin, CD68, B-cell lymphoma 2, a1-antitrypsin and CD10. Glypican 3 (GPC3), which is known to be a diagnostic marker for hepatoblastoma and hepatocellular carcinoma has recently been shown to be positive in a subset of UESL. Therefore, GPC3 staining is not a reliable marker to differentiate UESL from hepatoblastoma and hepatocellular carcinoma. Furthermore, hepatocyte paraffin 1, myogenin, CD34, C-kit (CD117), surfactant (PE10), anaplastic lymphoma kinase 1 (ALK-1), and S100 are negative in most cases. Individual markers are often not helpful in differentiating UESL from other liver tumors. Therefore, multiple immunostains are usually performed to help with the diagnosis. In practice, the negative markers are valuable to rule out the differential diagnoses. Hepatoblastoma and hepatocellular carcinoma are commonly positive for hepatocyte paraffin 1 antibody. Myogenin is usually positive in embryonal rhabdomyosarcoma. CD34 positivity is seen in solitary fibrous tumor and vascular neoplasms. Gastrointestinal stromal tumor is positive for both C-kit and CD34. Nuclear positive staining of C-kit in several UESL cases has been described by Kiani et al. [5]. It is important to differentiate this finding from the cytoplasmic staining of C-kit in gastrointestinal stromal tumor. ALK-1 is positive in anaplastic large cell lymphoma and inflammatory pseudotumor. Finally, negative staining for S100 and melanin markers is helpful to exclude melanoma and neural tumors. The extensive panels of immunohistochemical markers are helpful in diagnosing UESL. However, the antibody selection has to be based on demographic information, clinical history, and histologic findings. Because of the nonspecific immunophenotyped we recommend at least 2 or 3 commonly positive antibodies to confirm the diagnosis of UESL in addition to markers that are needed to rule out the differential diagnosis.
UESL ought to be differentiated from hepatoblastoma, embryonal rhabdomyosarcoma, hepatic mesenchymal hamartoma and hepatic echinococcosis. Hepaoblastoma, which mainly occurs in infants aged <3 years, is composed of primary hepatic parenchymal cells with small cellular heteromorphism, little karyokinesis, increased blood sinus and positive expression of endosomal membrane protein, vimentin and AFP revealed by immunohistochemistry. By contrast embryonal rhabdomyosarcoma mainly occurs in infants aged <6 years and the tumor are mainly composed of striated muscle maternal cells in different phases and primary mesenchymal cells. The myosin often exhibits strong positive expression by immunohistochemistry and transverse striation may be observed by preservative associated transient hyper fluorescence (PATH) staining. With hepatic mesenchymal hamartoma which mainly occurs in infants aged <1 year, the tumor is composed of a mucus matrix and a large number of star and fusiform primary mesenchymal cells whereas the tumor cells themselves do not exhibit heteromorphism. By contrast hepatic echinococcosis is a local parasitic disease that mainly occurs in adults aged 2040 years. Casoni and complement fixation tests are used to diagnose this disease and the positive rate is 90-95%, which has a critical diagnostic value. A case history combined with imaging analysis has been demonstrated to be beneficial for the discrimination of UESL. A previous study has revealed that UESL is often misdiagnosed as echinococcosis of the liver. Previous studies have demonstrated that cases of UESL have exhibited an amplification and deletion in chromosomes 1q, 5p, 8p and 12q and a translocation of 19q13 [4]. As well as mutation of the p53 gene. Therefore, the detection of molecular genetics is beneficial for the differential diagnosis of UESL. Previously prognosis of UESL had been poor. In 1990 Leuschner et al. reported a low survival rate (37%) of patients with UESL [7,8]. At that time management of UESL relied primarily on surgical resection. However, the prognosis has slowly improved as these patients are managed with multimodal treatment including radiation therapy and chemotherapy. Neoadjuvant chemotherapy is often helpful in unresectable cases. In additionpostoperative chemotherapy and radiation therapy are often reasonable options particularly in surgical cases with positive margins. Studies [9-11] have shown improved survival rates ranging from 70% to 100% in patients who were treated with multimodal therapy. The recurrence rate in UESL is higher during the first 2 years after surgery and the risk is higher with positive resection margins and cases with spontaneous or iatrogenic rupture of the hepatic lesion.
Conclusion
In conclusion, although UESL is predominantly seen in children and is a rare entity. Histological examination and immune-histochemical evaluation are vital as its clinical and radiological findings are often not specific. Timely diagnosis and multimodal treatment is needed to improve survival rates.
References
- Gao J, Fei L, Li S, Cui K, Zhang J, et al. (2013) Undifferentiated embryonal sarcoma of the liver in a child: A case report and review of the literature. Oncol let 5: 739-742.
- Juan P,Ornvold K (2015) Undifferentiated embryonal sarcoma of the liver: a concise review. Arch Pathol Lab Med 139: 269-273.
- Stocker JT, Ishak KG (1978) Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 42: 336-348.
- Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, et al. (2008) Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J HepatobiliaryPancreatSurg 15: 536-544.
- Kiani B, Ferrell LD, Qualman S, Frankel WL (2006) Immunohistochemical analysis of embryonal sarcoma of the liver. ApplImmunohistochemMolMorphol14:193-197.
- Wei ZG, Tang LF, Chen ZM, Tang HF, Li MJ (2008) Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J PediatrSurg43:1912-1919.
- Weinberg AG, FinegoldMJ (1983) Primary hepatic tumors of childhood. Hum Pathol14:512-537.
- Leuschner I, Schmidt D, Harms D (1990) Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry and literature review. Hum Pathol21:68-76.
- May LT, Wang M, Albano E, Garrington T, Dishop M, et al. (2012) Undifferentiated sarcoma of the liver: a single institution experience using a uniform treatment approach. J PediatrHematolOncol34:114-116.
- Plant AS, BusuttilRW, Rana A, Nelson SD, Auerbach M, et al. (2013) A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J PediatrHematolOncol35:451-455.
- Weitz J, Klimstra DS, Cymes K, JarnaginWR, D'Angelica M, et al. (2007) Management of primary sarcomas. Cancer109:1391-1396.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences