An Unusual Cause of Jaundice
Husain Taha, Ebtihal Al-Yusuf, Eman Al-Basri and Zahra Al-Yusuf
1Department of Endocrinology, Salmanyia Medical Complex, Bahrain
2Department of Radiology, Salmanyia Medical Complex, Bahrain
- *Corresponding Author:
- Taha H
Department of Endocrinology
Salmanyia Medical Complex, Bahrain
Tel: +973 39639960
E-mail:husaintaha74@yahoo.com
Received Date: November 21, 2018; Accepted Date: December 18, 2018; Published Date: December 21, 2018
Citation: Taha H, Al-Yusuf E, Al-Basri E, Al-Yusuf Z (2018) An Unusual Cause of Jaundice. Med Case Rep Vol. 4 No.4:89.
Abstract
Background: Jaundice has been reported in patients with Grave’s disease thyrotoxicosis and it can occur by different mechanisms. Case study: To describe a case of an 18-year-old male who presented with jaundice and thyrotoxicosis whom an extensive work up has excluded other causes of hepatic dysfunctionand he improved remarkably following treatment with radioactive iodine. Conclusion: Grave’s disease can cause jaundice and hepatic dysfunction in which treatment of thyrotoxicosis with radioactiveiodine could be a safe modality of therapy and it can cause resolution of both thyrotoxicosis and hepatic dysfunction.
Keywords
Jaundice; Thyrotoxicosis; Radioactive iodine
Introduction
Graves’ disease thyrotoxicosis classically presents with palpitations, tremor, weight loss, goiter and graves eyes disease. Rarely, thyrotoxicosis presents with painless jaundice and considerable liver dysfunction. A complex relationship exists between the thyroid gland and the liver. Thyroid hormones are important for normal hepatic function while the liver is important in the metabolism of thyroid hormone [1]. Jaundice and abnormalities in liver function tests in patients with thyrotoxicosis could be due to thyrotoxicosis itself, antithyroid drugs, concomitant autoimmune hepatitis, congestive heart failure, or unrelated viral hepatitis [2].
Management of patients with this presentation is challenging given that anti-thyroid drugs have been associated with drug induced liver injury. Thyroidectomy and radioactive iodine (RAI) therapy are well established alternatives, but each has its own contraindications.
We present an unusual case of hyperthyroidism associated hepatocellular pattern of liver dysfunction in which RAI were used and cause biochemical and clinical resolution of liver damage and thyrotoxicosis.
Case Report
An 18-year-old sickle cell trait male presented with two weeks of new onset painless jaundice and four weeks of fatigue, palpitation, hand tremor, sweating, and unintentional weight loss of 4 kg. He denied any history of fever, purities, or abdominal pain. He also denied any history of alcohol intake, intravenous drug use, smoking, travel, high risk sexual behavior, or blood transfusion. His family history was noncontributory.
Physical examination revealed a deeply icteric young male with a BMI of 24 kg/m2, resting tachycardia, hypertension, and fine tremor. He was having a goiter which was smooth, nontender, and without audible bruit or palpable nodules. There was no evidence of graves orbitopathy or pretibial myxedema. A cardiovascular examination was unremarkable except for tachycardia. Abdominal examination was normal with no tenderness, hepatosplenomegaly or other signs of chronic liver disease.
Laboratory tests showed a hepatocellular pattern of liver injury with elevated aspartate aminotransferase (AST), 102 U/L (reference range, 10 to 30 U/L), alanine aminotransferase (ALT), 225 U/L (reference range, 10 to 40 U/L), total bilirubin (TB), 355 μmol/L (reference range, 5 to 21 μmol/L), direct bilirubin (DB), 332 μmol/L (reference range, 0 to 5 μmol/L), globulin 37 g/L (reference range, 15 to 30 g/L), hepatic synthetic dysfunction with an international normalized ratio (INR), 1.38 (reference range, 0.9 to 1.20), and normal Alkaline phosphatase (ALP) and Gamma-glutamyl transferase (GGT). Thyroid stimulating hormone (TSH) was suppressed, 0.01 mI U/L (reference range, 0.25 to 5 mI U/L), with an elevated triiodothyronine (T3) level of 30 pmol/L (reference range, 2.5 to 7.8 pmol/L), and free thyroxin (T4) level, 79.8 pmol/L (reference range, 6.0 to 24 pmol/L) (Table 1).
Table 1 Serial changes in values of thyroid function and liver function tests.
| Test | At presentation | 1 month after RAI therapy | 3 months after RAI therapy | 2 months after thyroxin | Reference range |
|---|---|---|---|---|---|
| TSH | 0.01 | 0.01 | 55 | 5 | 0.25-5 mI U/L |
| FT4 | 79.8 | 23.7 | 2.3 | 20 | 6.0-24 pmol/L |
| T3 | 30 | 8.3 | 1 | 4 | 2.5-7.8 pmol/L |
| TB | 355 | 22 | 4 | 5 | 5-21 µmol/L |
| DB | 332 | 20 | 3 | 4 | 0-5 µmol/L |
| AST | 102 | 16 | 12 | 14 | 10-30 U/L |
| ALT | 225 | 36 | 30 | 34 | 10-40 U/L |
| ALP | 114 | 64 | 100 | 102 | 40-129 U/L |
| GGT | 21 | 25 | 24 | 25 | 10-71 U/L |
| INR | 1.38 | 1 | 1.1 | 1 | 0.9-1.20 |
| Globulin | 37 | 34 | 37 | 36 | 15-30 g/L |
TSH: Thyroid Stimulating Hormone; FT4: Free Thyroxine; T3: Triiodothyronine; TB: Total Bilirubin; DB: Direct Bilirubin; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; ALP: Alkaline Phosphatase; GGT: Gamma-Glutamyl Transferase; INR: International Normalized Ratio.
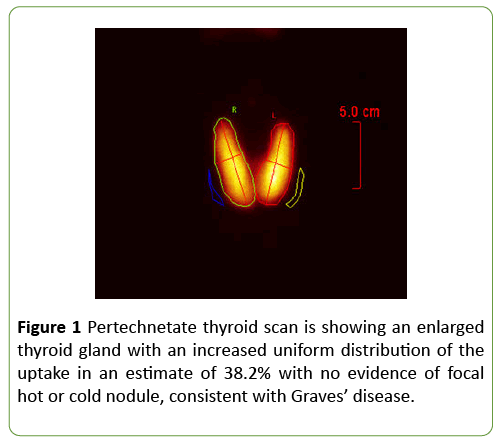
Pertechnetate thyroid scan was consistent with Graves’ disease (Figure 1). Extensive further testing excluded other causes of liver disease. Hepatitis A IgM antibodies, Hepatitis B surface antigen, Hepatitis B core IgM antibodies, Hepatitis C antibodies, Hepatitis E antibodies, Epstein-Barr virus (EBV) IgM and IgG antibodies, cytomegalovirus CMV IgG and IgM antibodies, anti-smooth muscle antibodies, antinuclear antibodies, anti-Liver kidney microsomal antibodies, antimitochondrial antibodies, ceruloplasmin, hemochromatosis screen, HIV antigen-antibody were all negative. Ultrasound of the abdomen and magnetic resonance cholangiopancreatography (MRCP) showed no biliary ductal dilatation, pancreatic, biliary, or liver masses, portal vein thrombosis or finding suggestive of primary sclerosing cholangitis. Echocardiogram was normal with ejection fraction of 64%.
Propranolol was commenced. Considering his hepatocellular pattern of liver derangement, Carbimazole and propylthiouracil were not initiated. Iodine -131 radioactive iodine therapy was arranged and received after 4 weeks. He made significant improvement clinically and biochemically and he is maintained on thyroxin 125 mcg daily, with regular follow up in the endocrine clinic. The follow up investigations are shown in Table 1.
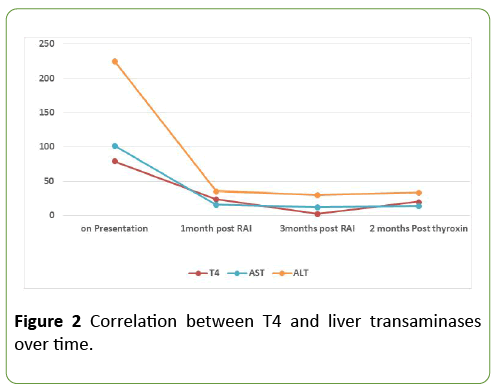
Figure 2 is showing the correlation between free thyroxin and liver transaminases over time.
Discussion
Patients with Graves’ disease (GD) usually present with common manifestations such as palpitations, tremors, heat intolerance, and weight loss. However, some patients may present with unusual gastrointestinal, hematologic, neurologic, and cardiopulmonary complications [3].
Hepatic dysfunction has been described in patients with hyperthyroidism since 1874 [4]. The hepatic derangement in patients with GD ranges from mild laboratory abnormalities without clinical features to overt hepatitis, with either hepatocellular or cholestatic injury [1]. Our patient had a hepatocellular pattern of liver impairment with raised direct bilirubin, AST, ALT, and globulin. In a retrospective study conducted on hospitalized patients about hepatic dysfunction in patients with thyrotoxicosis and not on anti-thyroid medications, 81% had some degree of hepatic dysfunction [5]. A cohort study conducted by Tiffany Y. and his colleagues on 1514 patients of thyrotoxicosis showed that the incidence of any biochemical liver tests abnormalities within 6 months of thyrotoxicosis was 39% [6]. Significant predictors of abnormal serum liver biochemical test result within 6 months of a diagnosis of untreated thyrotoxicosis were identified in this study and they were: serum TSH concentration <0.02 mI U/L, male gender, and African-American race. Biscoveanu and Hasinski have reported in their study of 30 patients with Grave’s disease that liver enzymes have different rates of elevation [2]. The reported increase in liver enzymes was as followed: ALP (33%), ALT (26%), GGT (24%), AST (17%), and total bilirubin (8%).
Different mechanisms for the hepatic damage in Grave’s disease have been reported. It can be induced by ischemic and hypoxic injury which resulted from the increase in metabolic rate and relative decrease in blood flow to the liver. Also, a direct toxic effect on hepatic tissues by thyroid hormones can be the contributory mechanism [7]. A common reason for jaundice in thyrotoxicosis is anti-thyroid drug induced liver injury. This was not the case with our patient as he was not previously diagnosed with thyrotoxicosis and had not received any anti-thyroid medications prior to his presentation. Other causes of liver dysfunction were excluded by extensive work up like viral hepatitis, congestive heart failure, and biliary obstruction. Moreover, improvement of liver function was only noticed after RAI ablation. This implies that the cause of jaundice and liver dysfunction was probably the thyrotoxicosis itself. Autoimmune hepatitis is one of the rare causes of jaundice in patients with Graves’ disease which has a strong association with autoimmune thyroid disease. The diagnosis of which needs liver biopsy which would be indicated if raised liver enzymes persists after treatment of thyrotoxicosis.
A review of the literature reveals only a few of case reports of thyrotoxicosis associated jaundice with almost all describing an initial treatment of thionamide or RAI therapy [7-10]. Management of hyperthyroidism in patients with raised liver enzymes is a clinical challenge because anti-thyroid drugs have been associated with drug induced liver injury [11]. Propylthiouracil usually causes hepatocyte damage while carbimazole and methimazole causes cholestasis. RAI therapy and thyroidectomy are well established alternatives, but these have their associated risks and contraindications. Thyroidectomy is relatively contraindicated in these patients since near normal thyroid function is required prior to surgery. RAI therapy is more reasonable choice for patients with hyperthyroidism and raised liver enzymes. RAI causes minimal harmful effects on surrounding tissues as it’s mainly concentrated in the thyroid gland [12]. The dose of radiation that is absorbed by the liver in the treatment of hyperthyroidism is very minimal [13]. In a recent publication by Renfei Wang and his colleagues, among 2385 Graves’ disease patients, liver dysfunction was present in 1552 patients and liver function was normalized in 77% of the cases 6 months after RAI [14].
Conclusion
Graves’ disease can present with hepatic dysfunction and jaundice without preexisting liver disease that complicates the therapeutic decision. Treatment with RAI ablation can lead to resolution of both hepatic dysfunction and hyperthyroidism.
References
- Malik R, Hodgson H (2002) The relationship between the thyroid gland and the liver. QJM: An International Journal of Medicine 95: 559-569.
- Biscoveanu M, Hasinski S (2000) abnormal results of liver function tests in patients with Grave's disease. Endocrine Practice 6: 367-369.
- Hegazi MO, Ahmed S (2012) Atypical clinical manifestations of Graves' disease: An analysis in depth. Journal of Thyroid Research 2012.
- Habershon SO (1874) Exophthalmic goiter, heart disease, jaundice, death. Lancet 1: 510-512.
- Elias RM, Dean DS, Barsness GW (2012) Hepatic dysfunction in hospitalized patients with acute thyrotoxicosis: a decade of experience. ISRN Endocrinology pp: 1-6.
- Lin TY, Shekar AO, Li N, Yeh MW, Saab S, et al. (2017) Incidence of abnormal liver biochemical tests in hyperthyroidism. Clinical endocrinology 86: 755-759.
- Chawla M, Bal CS (2008) Four cases of coexistent thyrotoxicosis and jaundice: Results of radioiodine treatment and a brief review. Thyroid 18: 289-292.
- Ding Y, Xing J, Qiu Z, Wang Y, Zhang Y, et al. (2015) Radioactive iodine therapy without recent anti-thyroid drug pretreatment for hyperthyroidism complicated by severe hyperbilirubinemia due to hepatic dysfunction: An experience of a Chinese Medical Center. Endocrine Practice 22: 173-179.
- Hull K, Horenstein R, Naglieri R, Munir K, Ghany M, et al. (2007) Two cases of thyroid storm-associated cholestatic jaundice. Endocrine Practice 13: 476-480.
- Klangjareonchai T (2012) An unusual case of hyperthyroidism associated with jaundice and hypercalcaemia. BMJ Case Reports 2012: bcr1120115076.
- Leo SD, Lee SY, Braverman LE (2016) Hyperthyroidism. The Lancet 388: 906–918.
- Emami B, Lyman J, Brown A, Cola L, Goitein M, et al. (1991) Tolerance of normal tissue to therapeutic irradiation. International Journal of Radiation Oncology, Biology & Physics 21: 109-22.
- Melo DR, Brill AB, Zanzonico P, Vicini P, Moroz B, et al. (2015) Organ dose estimates for hyperthyroid patients treated with 131I: An update of the thyrotoxicosis follow-up study. Radiation Research 184: 595-610.
- Wang R, Tan J, Zhang G, Zheng W, Li C (2017) Risk factors of hepatic dysfunction in patients with Graves’ hyperthyroidism and the efficacy of 131iodine treatment. Medicine 96: e6035.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences