WegenerÃÆâÃâââ¬Ãâââ¢s Granulomatosis Presenting as An Intracranial Hypertension Syndrome Treated with High Dose of Cyclophosphamide
Carmela Leone, Emanuele D’Amico, Nunzio Platania, Rosario Caltabiano, Mario Zappia, and Francesco Patti
DOI10.21767/2471-8041.100088
Carmela Leone, Emanuele D’Amico, Nunzio Platania, Rosario Caltabiano, Mario Zappia, and Francesco Patti*
Department of Medical Sciences, Surgicals and Advanced Technologies G.F. Ingrassia, Section of Neuroscience, University of Catania, Catania, Italy
- *Corresponding Author:
- Francesco Patti
Department of Medical Sciences
Surgicals and Advanced Technologies G.F. Ingrassia
Section of Neuroscience, University of Catania
Via Santa Sofia, Catania, Italy
Tel: +0039095372642
Fax: 0953782741-2900
E-mail: patti@unict.it
Received Date: November 27, 2017 Accepted Date: November 28, 2017 Published Date: November 30, 2017
Citation: Leone C, D’Amico E, Platania N, Caltabiano R, Zappia M, et al. (2017) Wegener’s Granulomatosis Presenting as An Intracranial Hypertension Syndrome Treated with High Dose of Cyclophosphamide. Med Case Rep Vol.4 No.1:53. doi: 10.21767/2471-8041.100088
Abstract
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's granulomatosis, is a rare form of vasculitis. In this disorder, small-sized blood vessels in the nose, sinuses, ears, lungs and kidneys become inflamed and damaged. Other areas may also be affected in some cases. It can also produce a type of inflammatory tissue known as a granuloma that's found around the blood vessels. Granulomas can destroy normal tissue. It is the result of inflammation within the tissues called granulomatous inflammation and blood vessel inflammation ("vasculitis"), which can damage organ systems.
Keywords
Wegener's granulomatosis; Central nervous system; Intracranial hypertension; Treatment; Cyclophosphamide
Introduction
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's granulomatosis, is a rare form of vasculitis. The areas most commonly affected by Wegener’s Granulomatosis include the sinuses, lungs, and kidneys, but any site can be affected. No one knows the cause of Wegener's granulomatosis. It is thought to be an autoimmune disorder in which the body's defense system attacks itself and destroys normal body tissue. Diagnosis of GPA is confirmed by detecting both abnormal cellular formations, called granulomas, and vasculitis. Treatment is directed toward stopping the inflammation process by suppressing the immune system. Anyone can get it, including children, but it's most common in middle-aged or older people. Wegener’s Granulomatosis can be very serious but, with medication, most people can keep it under control and live largely normal lives. Wegener’s Granulomatosis used to be called Wegener's granulomatosis. GPA can be hard to diagnose. It causes a range of symptoms and they are often similar to those of more common conditions.
Case Presentation
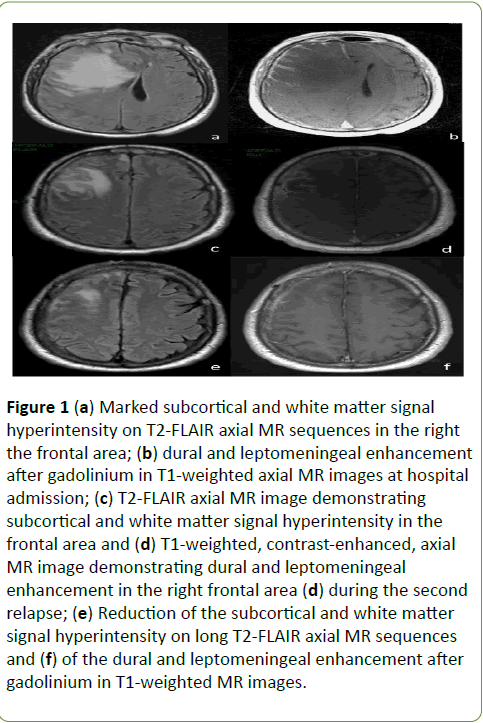
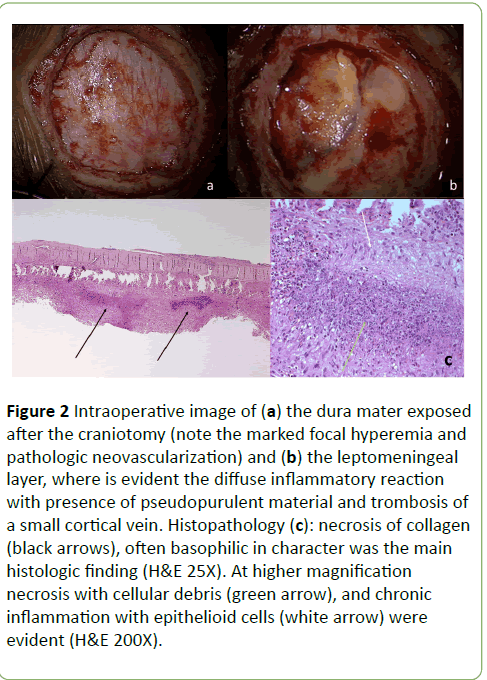
In October 2011, a 53-year-old white male experienced an increasingly severe and permanent left facial and cranial pain. Three months later, the intensity of pain increased while insomnia and behavioural changes occurred. He turned aggressive and irritable. The ear-nose-throat consultant prescribed a facial bones CT, which revealed spread inflammatory process of the nasal cavity and paranasal sinuses. A Para nasal sinuses biopsy showed hystopahological features of Wegener’s Granulomatosis (WG). He was treated with oral corticosteroids and azatioprin, reporting a complete recovery of the facial pain, although behavioural changes remained. In October 2012, he started to complain severe, progressive neck and cervical pain, followed by diplopia, left upper limb numbness and weakness, progressive confusional state, followed by vomiting and severe headache. When admitted to the Emergency Room of our Hospital the neurological examination showed a slight left hemiparesis. The brain Magnetic Resonance (MR) showed diffuse edema in right frontal with controlateral shift of midline structures and subfalcine herniation. The dura mater and pial-subarachnoid space showed contrast enhancement (Figures 1a and 1b). Laboratoristic and radiologic screening revealed only neutrophilic leukocytosis and non-specific signs of inflammation. Screening for autoantibodies, including antineutrophil cytoplasmic antibodies (c-ANCA e p-ANCA), and infectious conditions, including HIV, were negative. Hence, the patient underwent a dural and leptomeningeal biopsy which revealed histopathological findings suggestive of “WG of the central nervous system” (Figure 2).
Figure 1: (a) Marked subcortical and white matter signal hyperintensity on T2-FLAIR axial MR sequences in the right the frontal area; (b) dural and leptomeningeal enhancement after gadolinium in T1-weighted axial MR images at hospital admission; (c) T2-FLAIR axial MR image demonstrating subcortical and white matter signal hyperintensity in the frontal area and (d) T1-weighted, contrast-enhanced, axial MR image demonstrating dural and leptomeningeal enhancement in the right frontal area (d) during the second relapse; (e) Reduction of the subcortical and white matter signal hyperintensity on long T2-FLAIR axial MR sequences and (f) of the dural and leptomeningeal enhancement after gadolinium in T1-weighted MR images.
Figure 2: Intraoperative image of (a) the dura mater exposed after the craniotomy (note the marked focal hyperemia and pathologic neovascularization) and (b) the leptomeningeal layer, where is evident the diffuse inflammatory reaction with presence of pseudopurulent material and trombosis of a small cortical vein. Histopathology (c): necrosis of collagen (black arrows), often basophilic in character was the main histologic finding (H&E 25X). At higher magnification necrosis with cellular debris (green arrow), and chronic inflammation with epithelioid cells (white arrow) were evident (H&E 200X).
The patient started Cyclophosphamide (Cyc), at the dosage of 0.75 g/m2 body surface area, each other day, reaching the dosage of 6.5 g in ten days. After the second bolus of Cyc, he significantly improved; brain MR recorded a huge reduction of edema with partial remission of the dural contrast enhancement. The laboratoristic nadir, as suggested by Cyc use in aggressive forms of Multiple Sclerosis [1], was reached thirteen days after the first Cyc dose (WBC 2.43 × 103/mmc, lymphocytes 0.39 × 103/u). During the treatment, the patient had fever related to Staphylococcus epidermidis infection, which was promptly treated. At the discharge, he clinically and radiologically recovered, with the indication to continue treatment with monthly administration of Cyc. But 2 weeks after the discharge, he was re-admitted to our hospital for a refractory tonic-clonic status epilepticus. A new brain MR showed the reappearing of the dural contrast enhancement (Figure 2). Seven days later he was extubated, without complications and treated with intravenous methylprednisolone 1 g daily for 5 days reporting a progressive clinical and radiologic recovering (Figures 2c and 2d).
He was discharged and continued to be treated with Cyc for the following six months receiving a cumulative dose of 40 g. Clinical and radiologic (Figures 2e and 2f) evaluation over 2 years was stable, while recently serum c-ANCA antibodies were detected. The patient goes well with maintenance therapy (methotrexate).
Discussion
WG is a rare, necrotising, systemic small vessel vasculitis, potentially affecting every organ or tissue [2], with an autoimmune pathogenesis, strongly supported by its association with typical antibody c-ANCA [3]. Nervous system (NS) is involved in about 20-55% of cases [4]. A significant proportion of patients presents with peripherial NS involvement, while about 10-45% of patients presents with central NS involvement [5]. Meningeal involvement is rare, with reported frequencies of only 1% [4] and is thought to result from granulomatous tissue spreading out from the nasal-paranasal cavities and invading neighbouring structures such as the meninges and cranial nerves. Our case is the first presenting with an “intracranial hypertension syndrome”, confirmed by the presence of widespread aedema, marked effect mass and displacement of the controlateral midline structures and subfalcine erniation.
Since MR imaging of brain abscesses, neoplasms and metastasis can result in similar patterns, the diagnosis required confirmation by brain biopsy. Due to failing treatment with oral steroids, our patient was treated with high dose of Cyc alone. The CNS lesions responded well and regressed quickly, even if partially. Since 1973, when Fauci and Wolff published their experience with Cyc 2 mg/Kg/day, long-term experience provided greater evidence for its efficacy (80% of survival rate, 91% of significant improvement and 75% of complete remission) [6]. However, extended follow-up also demonstrated that disease relapse in about 50% of patients.
Conclusion
The peculiarity of our case is the use of Cyc we did (6.5 gr of Cyc in ten days, followed by monthly Cyc for six months) based on the evidences of high dose Cyc effectiveness in other autoimmune disease [7]. Our patient experienced a disease relapse after one month the last dose of Cyc and just before the next scheduled first monthly dose, supporting the huge importance of immunosuppressive treatment in this patient. The collective experience supports the use of Cyc in severe active WG, limited strictly to that period (typically 3-6 months) necessary to gain disease improvement or remission; then Cyc should be switched to a maintenance agents (methotrexate or azathioprine). We conclude that in all cases of neurological symptoms occurring in patient with WG, an intracranial localization of the disease should be considered. Dural and leptomeningeal biopsy should be considered as an important diagnostic tool before to start a specific treatment. Cyc treatment remains one of the most significant treatments to halt a cerebral disease relapse.
Author's disclosures
Dr. D’Amico won a grant from Merk Serono. Dr. Patti has served on the scientific advisory board for Teva, Biogen-Idec, Bayer-Schering, Novartis and has received honoraria as a speaker for Teva, Biogen, Merck-Serono, Bayer-Schering, Genzyme/Sanofi, Novartis. Dr. Leone has served on the scientific advisory board for Merk and Genzyme, and received grants for congress from Merk Serono, Biogen-Idec and from the Department GF Ingrassia. Dr. Zappia has received grants from Novartis, Biogen, Merck Serono, Teva e Almirall and has received honoraria from Sanofi-Genzyme. Dr. Platania and Dr. Caltabiano have nothing to disclose.
References
- Patti F, Cataldi ML, Nicoletti F, Reggio E, Nicoletti A, et al. (2001) Combination of cyclophosphamide and interferon-beta halts progression in patients with rapidly transitional multiple sclerosis. J Neurol Neurosurg Psychiatry 71: 404-407.
- Duna GF, Galperin C, Hoffman GS (1995) Wegener’s granulomatosis. Rheum Dis Clin North Am 21: 949–986.
- Van der Woude FJ, Rasmussen N, Lobatto S (1985) Autoantibodies against neutrophils and monocytes: Tool for diagnosis and marker of disease activity in Wegener’s granulomatosis Lancet 1: 425–429.
- Nishino H, Rubino FA, DeRemee RA, Swanson JW, Parisi JE (1993) Neurological involvement in Wegener’s granulomatosis: An analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol 33: 4-9.
- Holle JU, Gross WL (2011) Neurological involvement in Wegener’s Granulomatosis. Curr Opin Rheumatol 23: 7-11.
- Fauci AS, Haynes BF, Katz P (1983) Wegener’s granulomatosis: Prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 98: 76-85.
- Weiner HL, Cohen JA (2002) Treatment of multiple sclerosis with cyclophosphamide: Critical review of clinical and immunologic effects. Mult Scler 8: 142-154.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences