Proteinuria Associated with Thyroid Disorders - Cases Report and Literature Review
Yaxin Hao, Xiaomei Wu, Xiao Huang and Zheng Tang
DOI10.21767/2471-8041.100070
Yaxin Hao1, Xiaomei Wu2, Xiao Huang2 and Zheng Tang1*
1National Clinical Research Center of Kidney Diseases, Jingling Hospital, Nanjing University School of Medicine, Nanjing, P.R. China
2National Clinical Research Center of Kidney Diseases, Jinling Hospital, Nanjing University School of Medicine, Nanjing, P.R. China
- *Corresponding Author:
- Zheng Tang
National Clinical Research Center of Kidney Diseases Jinling Hospital, Nanjing University School of Medicine 305 East Zhongshan Road, Nanjing 210002, P.R. China
Tel: +86-25-80863761
E-mail: tang_dr@163.com
Received Date: July 31, 2017, Accepted Date: August 12, 2017, Published Date: August 14, 2017
Citation: Hao Y, Wu X, Huang X, Tang Z (2017) Proteinuria Associated with Thyroid Disorders-Cases Report and Literature Review. Med Case Rep, 2017, Vol.3 No.4: 35. doi:10.21767/2471-8041.100070
Abstract
Background: The interactions between thyroid and kidney have been reported mainly in the patients with proteinuria coexistent with autoimmune thyroiditis. The proposed mechanisms focus on direct effects of thyroid hormone on the hemodynamics and morphology of kidney and indirect effects through deposition of immune complexes consisting of thyroid component.
Case presentation: Herein we describe two cases presenting with proteinuria associated with thyroid disorder. Both patients presented with nephrotic-range proteinuria and hematuria but were not responsive to immunosuppression therapy. Immunopathology demonstrated the deposits of multiple immunoglobulins and complements along glomerular basement membrane and mesangium. One case presented with mesangial proliferative glomerulonephritis (MsPGN) in the context of thyroid papillary carcinoma. After removal of tumor, the urine protein disappeared. This is the first report about the MsPGN related to thyroid tumor. The other case was diagnosed as MN secondary to Hashimoto’s thyroiditis. More interestingly, both patients suffered relapse of the proteinuria accompanied by opposed disturbance of thyroid hormone at the follow-up. Once the thyroid hormone was corrected, the renal function improved.
Conclusion: This study proposes a possibility that a hormone-cytokine axis connecting these two organs mediates the changes of microenvironment in kidney with the thyroid hormone change, and then influence the renal function.
Keywords
Proteinuria; Glomerulonephritis; Thyroid disorders; Thyroid hormone
Abbreviations
MN: Membranous Nephropathy, MSPGN: Mesangial Proliferative Glomerulonephritis, AT: Autoimmune Thyroiditis: C1q: Complement 1q, C3: Complement 3, C4: Complement 4; IgA: Immunoglobulin A, IgG: Immunoglobulin G, IgM: Immunoglobulin M, FT3: Free Triiodothyronine; FT4: Free Thyroxine; TSH: Thyroid Stimulating Hormone; Anti-PLA2R: Anti-Phospholipase A2 Receptor Antibody; MCD: Minimal Change Disease; FSGS: Focal Segmental Glomerulosclerosis; MsPGN: Membranoproliferative Glomerulosclerosis; IgAN: Immunoglobulin A Nephritis; CTLA-4: Cytotoxic TLymphocyte Antigen 4; RAAS: Renin–Angiotensin– Aldosterone System; VEGF: Vascular Endothelial Growth Factor.
Introduction
The interplays between thyroid and kidney have been recognized in many disease states. Thyroid dysfunction can influence kidney through the immune-mediated pathway and thyroid hormones [1]. The thyroid disorder and coincident nephropathy have been reported mainly in the patients presented with proteinuria and autoimmune thyroiditis (AT) [2-7]. In this situation, the development and deposition of immune-complex in the glomerular is the key point in the onset of nephropathy. On the other hand, thyroid hormone may influence glomerular and tubular functions through prerenal and intrinsic renal effects. In patients with idiopathic nephritic syndrome, the treatment combines steroids with low-dose levothyroxine achieved improvement in shorter time compared with patients treated with steroids only [8]. In our two cases, one patient developed MsPGN secondary to thyroid cancer; the other developed the membranous nephropathy associated to Hashimoto’s thyroiditis. This is the first case of MsPGN associated to thyroid cancer. Besides, both patients developed relapse of proteinuria accompanied by opposite thyroid hormone states, namely hypothyroidism and hyperthyroidism. And when the thyroid hormone state was corrected, the proteinuria disappeared. We proposed that a hormone-cytokine axis may connect the kidney and thyroid and then mediate the renal local environment.
Case Presentation
Case 1
A 29 year-old man was admitted to our department due to a progressive edema with proteinuria of 2.3 g/24 h. Almost 1 year before his admission, he has received steroids therapy because of nephrotic-range proteinuria (4.7 g/24 h) and low serum albumin levels (30.8 g/L). But edema and proteinuria relapsed and maintained during steroids tapering. On admission, blood pressure was 137/100 mmHg, pulse was 112/min. Physical examination revealed enlargement of thyroid gland with a nodule on the right lobe and edema in the legs. Laboratory results revealed low serum albumin and high Cholesterol. Urinalysis revealed hematuria (red blood cell count 1.33 million /L) and proteinuria (protein excretion 1.51 g/24 h). Thyroid hormones were within normal range and thyroid associated antibodies were negative. Other serological examinations for autoantibodies, infection, liver and kidney function were normal. The renal biopsy revealed mild mesangial proliferative lesion and thickness of Bowman’s capsule without thickening of the glomerular basement membrane or spike. Immunopathology showed glomerular capillary walls and mesangium had +1 for IgG, IgA, IgM, C3, C4 and C1q in a diffuse, granular pattern. IgM and C3 were + in tubular epithelial cells. The renal biopsy demonstrated mild mesangial proliferative glomerulonephritis (MsPGN). At the same time, thyroid examination demonstrated a 14 × 10 mm nodule on and the right side of thyroid gland with decreased uptake of radioactive tracer, which was confirmed as papillary carcinoma by biopsy. Finally, total thyroidectomy of right lobe was performed with 40% of thyroid tissues left. Then Lthyroxine was administrated at a dose of 75 ug/day after surgery. He was also prescribed benazepril and losartan for proteinuria and moderate hypertension. After surgery, the urine protein disappeared. And annual examination didn’t show anything abnormal until 2014.
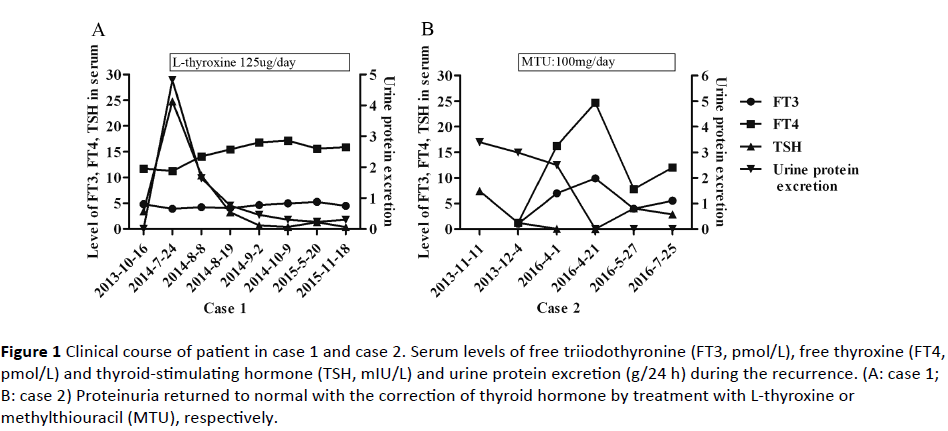
In the July of 2014, he readmitted to our department because of heavy proteinuria (4.82 g/24h). The antiphospholipase A2 receptor antibody (anti-PLA2R) test was negative. The thyroid analysis was consistent with subclinical hypothyroidism, as free triiodothyronine (FT3) 3.94 pmol/L (3.8-6.0 pmol/L, free thyroxine (FT4) 11.26 pmol/L (7.5-21.1 pmol/L), thyroid stimulating hormone (TSH) 24.77 mIU/L (0.34-5.6 mIU/L). Anti-thyroglobulin antibody was negative and thyroid ultrasonography of the left thyroid tissue was normal. Then L-thyroxine was increased to 125 ug/day gradually. With serum TSH decreased, the urine protein dropped. The clinical course was shown in Figure 1A. Till now, 1 year and a half after the recurrence, neither a relapse of hypothyroidism nor a flare-up of nephrotic syndrome was observed.
Figure 1: Clinical course of patient in case 1 and case 2. Serum levels of free triiodothyronine (FT3, pmol/L), free thyroxine (FT4, pmol/L) and thyroid-stimulating hormone (TSH, mIU/L) and urine protein excretion (g/24 h) during the recurrence. (A: case 1; B: case 2) Proteinuria returned to normal with the correction of thyroid hormone by treatment with L-thyroxine or methylthiouracil (MTU), respectively.
Case 2
A 22-year-old female was admitted to hospital on December 3, 2013, for further examination of proteinuria and hypothyroidism, which was incidentally discovered at a mass examination 3 months ago. At the examination, urinalysis showed 3+ for protein and hematuria. The thyroid function examination revealed hypothyroidismso the L-thyroxine was given at a dose of 100 ug/day immediately. During the 3 months prior to admission, urinary protein fluctuated at 2+~3+. Upon admission, physical examinations showed a moderate and bilateral enlargement of the thyroid gland. Urinalysis showed 3+ for hematuria and proteinuria with protein excretion 3.4 g/24 h. Serum albumin was 26 g/L with cholesterol 7.53 mmol/L. Liver and kidney function were normal. Thyroid function tests revealed euthyroidism. The serum anti-thyroglobulin antibody was positive with an extremely high titer (> 4000 IU/ml, normal <115) and antimicrosomal antibody was positive at a relatively lower titer (513.6 IU/ml, normal <34). At the same time, anti-thyroid stimulating hormone receptor antibodies were negative. Other serological examinations for autoantibodies, complement and infection were normal. Anti-PLA2R was negative. A renal biopsy was performed and the result was consistent with membranous nephropathy, stage II. Light microscopy showed glomeruli with mildly diffuse thickening of the basement membranes and occasional spikes on the sub-epithelial side of the membranes. There was also a focal and segmental increase in meningeal cells and matrix. The renal interstitium showed inflammatory cell infiltration. Immunofluorescence studies showed deposits of IgG (3+), IgA (+), IgM (+/-), C3 (2+), C4 (2+) and C1q (2+) along glomerular capillary walls and mesangium. IgG subclasses test in biopsy showed a predominant deposit of IgG1 (3+). Therefore, the diagnosis of MN secondary to Hashimoto’s disease was made. And treatment with levothyroxine and prednisone at a dose of 40 mg/day were commenced. But the urinary protein maintained at +~2+ for almost 6 months with therapy of high dose of steroids. But soon proteinuria relapsed. At the same time, the serologic examination indicated hyperthyroidism. Then the patient was prescribed methylthiouracil for hyperthyroidism as well as prednisone and irbesartan for proteinuria. Then the proteinuria disappeared accompanied by the correction of thyroid function (Figure 1B).
Discussion
The presented cases demonstrated an association between glomerulonephritis and thyroid dysfunction, which has been reported mainly in the cases of AT and coincident proteinuria. The most common glomerular lesion was membranous nephropathy, followed by MCD, FSGS, MPGN, IgAN and so on [1,2]. While, the tumors involved in glomerulonephritis mainly focused on the hematological malignancies and some solid tumor, like lung, rarely involved thyroid gland [9]. Our case provides additional evidence for the association between glomerulonephritis and thyroid tumor.
Whether and how thyroid disorders participate in the renal dysfunction is poorly understood. But the tendency to certain glomerulonephritis may indicate a common pathway in the pathogenesis of both diseases, in which environmental and genetic factors are involved. As immune-related diseases, impaired T cell function has been postulated to play a pathogenic role. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) expressed on activated T cells is implicated in the immune response. The +49A/G polymorphism in CTLA-4 is related to HT [10]. while the +49GG genotype of the +49A/G SNP in the CTLA-4 gene is associated with the risk for nephrotic kidney diseases [11]. Genetic predisposition may contribute to the concurrence of both diseases. Additionally, some virus, such as the human parvovirus B19 and hepatitis C virus have been thought to participate in the onset of thyroid disorders and nephropathy [12].
Immune complexes deposition plays a pivotal role in the pathogenesis of glomerulonephritis. There are two concepts for the deposition of immune complexes composed of thyroid component in glomerulonephritis, namely in situ immune complex formation [3,4] and circulating immune complex [5]. In this situation, thyroid tissue was postulated to serve as an excellent source of continuous antigen for the formation of immune complexes. It may explain why some patients developed proteinuria after radioactive iodine therapy while some patients with AT and proteinuria may benefit from the thyroidectomy [6,7]. For our first case, proteinuria accompanied by edema was not sufficiently prevented until thyroidectomy therapy. However, it is noteworthy that the relationship between the thyroid specific antibodies has been reported to be independent of renal function and proteinuria [2].
Additionally, thyroid hormone plays an important role in the maintenance of renal development and physiology. Both hyperthyroidism and hypothyroidism can influence cardiac output, vasodilatation-to-vasoconstriction ratio as well as activity of the renin–angiotensin–aldosterone system (RAAS) and then influence renal blood flow and GFR [1]. Additionally, renal biopsy in hypothyroid patients showed thickening of the GBM and tubular basement membrane, increased meningeal matrix, as well as cytoplasmic inclusions in renal tubular epithelial cells [13]. These atypical morphology changes may account for the proteinuria and hematuria observed with hypothyroidism.
Most strikingly, our 1st patient showed that serum levels of TSH exhibited a significantly positive correlation with urine excretion of protein (p<0.0001, R2=0.0.98 by Pearson correlation analysis), during his recurrence. A cross-sectional survey in type 2 diabetes patients also demonstrated a statistically significant association between serum TSH level and albuminuria. And serum TSH was considered as an independent risk factor of albuminuria [14]. The pathological mechanisms for this association are not clear. And it’s difficult to dissect if changes of renal function are directly related to the TSH or if both are independently caused by hypothyroidism. There are some assumptions for this situation. Patients who developed AT may form autoantibodies against thyroglobulin, thyroid peroxidase or megalin in a TSHdependent manner. When the level of TSH is corrected by the L-thyroxine, expression of megalin and formation of antibodies are stopped [4]. As known, megalin was identified as the autoantigen in Heymann nephritis. But unlike the rat mimics, human podocytes do not express megalin. Moreover, endothelial dysfunction was observed in the patients with subclinical hypothyroidism [15] which may be related to reduce vascular endothelial growth factor (VEGF) observed in hypothyroid state [16]. The loss of VEGF from the podocytes could influence the fenestrated phenotype of endothelium which is necessary for the functioning of the glomerular filtration barrier [17]. But in contrary, the VEGF level is higher in active nephrotic syndrome than those in remission [18]. An animal model highly expressing VEGF developed the FSGS-like injuries [19]. Hence, we proposed a possibility that some molecules like VEGF may form a hormone-cytokine axis to involve in the pathogenesis of proteinuria related to thyroiditis.
Conclusion
So far, no studies have established the exact molecular pathogenesis of the renal lesion associated with thyroid dysfunction. Herein, we proposed a possibility that a hormone-cytokine axis, including the activity of RAAS and level of small molecular, eg. VEGF, knit a network to mediate the change of microenvironment in kidney as the thyroid hormone change and then influence the renal function. Whatever, we deem it’s necessary to screen for and manage secondary factors and concomitant conditions when dealing with patients with kidney injuries.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Authors' Contributions
TZ was the primary physician who cared for the patient. HX and WXM were involved in patient care and contributed academically. HYX designed the case report and was a major contributor in writing the manuscript. TZ critically revised the manuscript. All authors read and approved the final manuscript.
References
- Basu G, Mohapatra A (2012) Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 16: 204.
- Kocak G, Huddam B, Azak A, Ortabozkoyun L, Duranay M (2012) Coexistent findings of renal glomerular disease with Hashimoto's thyroiditis. Clin Endocrinol (Oxf) 76: 759-762.
- Shima Y, Nakanishi K, Togawa H, Obana M, Sako M, et al. (2009) Membranous nephropathy associated with thyroid-peroxidase antigen. Pediatr Nephrol 24: 605-608.
- Illies F, Wingert AM, Bald M, Hoyer PF (2004) Autoimmune thyroiditis in association with membranous nephropathy. J Pediatr Endocrinol Metab 17: 99-104.
- Jordan SC, Buckingham B, Sakai R, Olson D (1981) Studies of immune-complex glomerulonephritis mediated by human thyroglobulin. NEJM 304: 1212-1215.
- Becker BA, Fenves AZ, Breslau NA (1999) Membranous glomerulonephritis associated with Graves' disease. Am J Kidney Dis 33: 369-373.
- Hasnain W, Stillman IE, Bayliss GP (2011) Minimal-change renal disease and Graves' disease: a case report and literature review. NDT plus.4: 96-98.
- Guo QY, Zhu QJ, Liu YF, Zhang HJ, Ding Y, et al. (2014) Steroids combined with levothyroxine to treat children with idiopathic nephrotic syndrome: a retrospective single-center study. Pediatr Nephrol 29:1033-1038.
- Lien YH, Lai LW (2011) Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol 7: 85-95.
- Feng M, Zhang FB, Deng HR (2013) The CTLA4 +49A/G polymorphism is associated with an increased risk of Hashimoto's thyroiditis in Asian but not Caucasian populations: an updated meta-analysis. Endocrine 44: 350-358.
- Spink C, Stege G, Tenbrock K, Harendza S (2013) The CTLA-4 +49GG genotype is associated with susceptibility for nephrotic kidney diseases. Nephrology Dialysis Transplantation 28: 2800-2805.
- Fallahi P, Ferrari SM, Vita R, Benvenga S, Antonelli A (2016) The role of human parvovirus B19 and hepatitis C virus in the development of thyroid disorders. Rev Endocr Metab Disord 17: 529-535.
- Salomon MI, Discala V, Grishman E, Brener J, Churg J (1967) Renal lesions in hypothyroidism - A study based on kidney biopsies. Metabolism 16: 846-850.
- Yasuda T, Kaneto H, Kuroda A, Yamamoto T, Takahara M, et al. (2011) Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract 94: e75-77.
- Dagre AG, Lekakis JP, Protogerou AD, Douridas GN, Papaioannou TG, et al. (2007) Abnormal endothelial function in female patients with hypothyroidism and borderline thyroid function. Int J Cardiol 114: 332-328.
- Schmid C, Brandle M, Zwimpfer C, Zapf J, Wiesli P (2004) Effect of thyroxine replacement on creatinine, insulin-like growth factor 1, acid-labile subunit, and vascular endothelial growth factor. Clin Chem 50: 228-231.
- Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, et al. (2008) VEGF inhibition and renal thrombotic microangiopathy. NEJM 358: 1129-1136.
- Cheong HI, Lee JH, Hahn H, Park HW, Ha IS, et al. (2001) Circulating VEGF and TGF-beta 1 in children with idiopathic nephrotic syndrome. J Nephrol 14: 263-269.
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, et al. (2003) Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707-716.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences