Endoscopic Biological Glue Injection for Treating Multiple Staple-Line Leaks and Dehiscence Following Laparoscopic Sleeve Gastrectomy ÃÆâÃâââ¬Ãâââ¬Å A Case Report and Review of the Literature
Leonid Maizels, Ahmad Assalia, Iyad Khamaysi and Ahmad Mahajna
DOI10.21767/2471-8041.S1-001
Leonid Maizels1, Ahmad Assalia1, Iyad Khamaysi2 and Ahmad Mahajna1*
1Department of General Surgery, The Division of Advanced Laparoscopic and Bariatric Surgery, Rambam Health Care Center, Israel
2Department of Gastroenterology and the Advanced Endoscopy Procedures Unit, Rambam Health Care Campus and Bruce Rappaport Faculty of Medicine, Technion Israel Institute of Technology, Haifa, Israel
- *Corresponding Author:
- Mahajna Ahmad
Department of General Surgery, The Division of Advanced Laparoscopy and Bariatric Surgery
Rambam Health Care Center, P.O.B 9602, Haifa 31096, Israel
Tel: 972-4-854-1832
Fax: 972-4-854-2898
E-mail: a_mahajna@rambam.health.gov.il
Received Date: December 20, 2017; Accepted Date: December 26, 2017; Published Date: December 28, 2017
Citation: Maizels L, Assalia A, Khamaysi I, Mahajna A (2018) Endoscopic Biological Glue Injection for Treating Multiple Staple-Line Leaks and Dehiscence Following Laparoscopic Sleeve Gastrectomy – A Case Report and Review of The Literature. Med Case Rep Vol.4 No. S1:001. DOI: 10.21767/2471-8041.S1-001
Abstract
In the past decade laparoscopic sleeve gastrectomy (LSG) has become the primary restrictive procedure in bariatric surgery. LSG offers an excellent outcome with regards to weight loss and comorbidity reduction. LSG is a restrictive procedure without the mal-absorptive component present in other bariatric procedures. It involves resection of two-thirds of the stomach to provide increased satiety and decreased appetite.
Sleeve gastrectomy for weight loss was first described by Marceau in 1993 as a component of biliopancreatic diversion. Laparoscopic sleeve gastrectomy (LSG) was performed as a component of biliopancreatic diversion with duodenal switch (BPD-DS) in 2000 by Ren et al. and subsequently used as the initial stage of a two-staged approach for super-morbidly obese patients.
Here, we describe a case of a an 18-year-old male presented to the emergency department complaining of a 3-day lasting abdominal pain, a week after undergoing LSG with an otherwise uneventful post-operative period.
Keywords
Laparoscopy; Bariatric surgery; Sleeve gastrectomy; Leak; Fibrin glue
Introduction
During the past decade laparoscopic sleeve gastrectomy (LSG) is becoming the primary restrictive procedure in bariatric surgery [1], and the number of operations performed is constantly increasing. LSG offers an excellent outcome with regards to weight loss and comorbidity reduction, a relatively simple surgical technique, and lower complication rates as compared with other procedures such as the Roux-en-y gastric bypass (RYGB) or biliopancreatic diversion with or without a duodenal switch procedure [1-4].
Overall complication rates following LSG may reach approximately 20% [5,6], including staple-line disruption and leakage, bleeding, stricture, and gastro-esophageal reflux [1,5-7]. Leaks are probably the most feared complication following LSG, with the majority occurring near the gastroesophageal junction (GEJ) [2,8,9].
Additional risk factors for leak development include inadequate sleeve-sizing, the presence of a stricture, local infection or ischemia, and performing the operation secondarily, following a previous bariatric procedure [1,2,4,5,10].
Staple-line leaks presentation may range from slight symptoms to overt peritonitis and sepsis. Although of potential concern to both patients and surgeons, while many treatment options are available, no proven protocols or guidelines currently offer a well-standardized approach to treat this potentially fatal complication.
We hereby report the case of conservative endoscopic treatment using Evicel fibrin glue for treating a patient with multiple leak sites and staple-line dehiscence a week after undergoing LSG.
Case Report
An 18-year-old male presented to the emergency department complaining of a 3-day lasting abdominal pain, a week after undergoing LSG with an otherwise uneventful postoperative period.
Upon admission, the patient was hemodynamically stable, with mild tachycardia but no fever. His physical examination was significant for diffuse abdominal pain, with no signs of peritonitis.
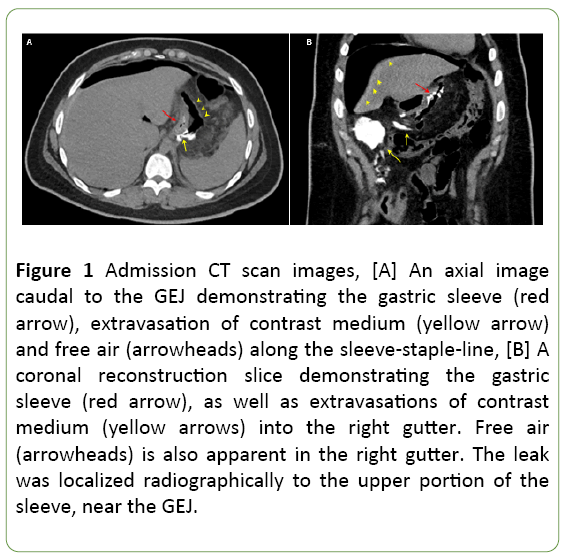
Laboratory tests were drawn. Complete blood count demonstrated marked leukocytosis (29 K/ul) with a left-shift. A computed tomography (CT) scan was performed demonstrating free, non-contained contrast medium leak into the right gutter, with fluid and free air present in the abdomen (Figure 1).
Figure 1: Admission CT scan images, [A] An axial image caudal to the GEJ demonstrating the gastric sleeve (red arrow), extravasation of contrast medium (yellow arrow) and free air (arrowheads) along the sleeve-staple-line, [B] A coronal reconstruction slice demonstrating the gastric sleeve (red arrow), as well as extravasations of contrast medium (yellow arrows) into the right gutter. Free air (arrowheads) is also apparent in the right gutter. The leak was localized radiographically to the upper portion of the sleeve, near the GEJ.
Based on these clinical and imaging findings, an exploratory laparoscopy was performed. During operation a leak was noted at the upper sleeve portion, near the GEJ, with a small amount of turbid fluid draining into the right gutter. Multiple peritoneal washes were preformed, and 3 drains were placed (1 on each side of the sleeve and 1 pelvic drain).
The drains adjacent to the leak were active draining turbid fluid. The pelvic drain produced only mild serous excretions and was subsequently removed.
The patient was placed on total parental nutrition (TPN). Nine days following the patient’s admission, an upper endoscopy with a concomitant fluoroscopy was performed to evaluate the leak site.
Our group has recently gained substantial experience using this technique (later described in detail) allowing the evaluation and minimally-invasive treatment of staple-line leaks.
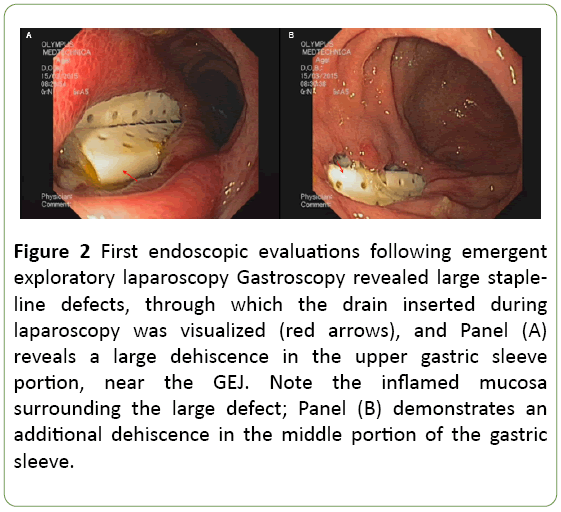
Endoscopy revealed a fistula 1 cm in diameter just distal to the GEJ, and 2 additional wide openings each of 2-3 cm in length, appearing as a staple-line dehiscence through which, the drains were visualized (Figure 2).
Figure 2: First endoscopic evaluations following emergent exploratory laparoscopy Gastroscopy revealed large stapleline defects, through which the drain inserted during laparoscopy was visualized (red arrows), and Panel (A) reveals a large dehiscence in the upper gastric sleeve portion, near the GEJ. Note the inflamed mucosa surrounding the large defect; Panel (B) demonstrates an additional dehiscence in the middle portion of the gastric sleeve.
Since the patient was stable and asymptomatic, it was decided to maintain conservative treatment and reevaluate his condition clinically and endoscopically in a one-week interval. The following upper endoscopy demonstrated substantial shrinkage of the defects with the initiation of approximation and closure.
Therefore, the patient was discharged after 19 days of hospitalization while on TPN and mild oral fluid intake, feeling well, and presenting normal laboratory findings. Two weeks later, he was electively re-hospitalized for endoscopic reevaluation and a potential adhesion attempt.
An upper endoscopy combined with fluoroscopy was performed for the 3rd time, revealing further decrease in defect sizes. An adhesion attempt was performed using 5 ml fibrin sealant, (Human) Evicel® (Johnson & Johnson), injected to the upper defect adjacent to the GEJ.
Post-procedure hospitalization was uneventful, and oral liquid intake was reestablished in two days. Nevertheless, turbid fluid was still draining from one of the drains (left), while the other (right) was inactive, prompting its removal.
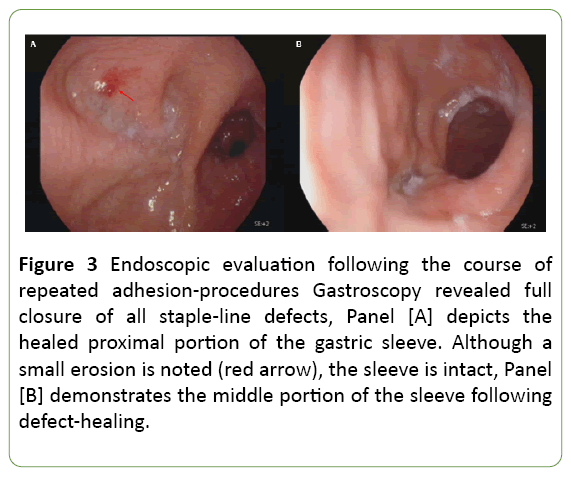
Subsequently, the patient was planned for repetitive elective adhesion attempts fortnightly. In practice, overall 4 additional adhesion attempts were performed during the actual course of 3.5 months with a gradual process of fistulaclosure following repeated adhesions (Figure 3).
Figure 3: Endoscopic evaluation following the course of repeated adhesion-procedures Gastroscopy revealed full closure of all staple-line defects, Panel [A] depicts the healed proximal portion of the gastric sleeve. Although a small erosion is noted (red arrow), the sleeve is intact, Panel [B] demonstrates the middle portion of the sleeve following defect-healing.
The treatment course was not uneventful, complicated by several occasions of intercurrent illnesses. Additionally, following one of the adhesion attempts, fever was documented briefly, but workup was negative.
Two weeks after the final procedure, the patient was readmitted due to spontaneous withdrawal of the left (and only) drain, accompanied by abdominal pain and fever.
A CT scan was performed demonstrating no further leak or other abnormalities. Fever was not recorded during hospitalization, blood workup was normal, and the patient was eventually discharged resuming normal oral intake. During a 10-month follow-up, no evidence of leak or fistula was noted, and the patient resumed normal daily life.
Adhesion technical description
A combined endoscopic and fluoroscopic approach is used to evaluate and treat staple-line leaks. The leak orifice is first detected during endoscopy. Subsequently, the fluoroscopic approach is utilized to percutaneously insert an applicator (e.g. via a drain) towards the leak-orifice. The drain is then removed, and a catheter is introduced over the applicator. Subsequently, 5 ml of fibrin sealant (Human) Evicel® (Johnson & Johnson) is injected via the catheter directly into the cavity adjacent to the leak-site, while sealant entry into the gastric cavity is visualized endoscopically. Sealant application is maintained during catheter withdrawal occluding the proximal tract. Finally, a pigtail drain is left in place to allow monitoring of continuous leakage and subsequent repeated adhesions if required.
Discussion
Although regarded as a relatively safe operation, LSG may harbor an overall complication rate of up to 20% [5,6]. Leaks are probably the most dreaded complication carrying a substantial risk to patients and presenting a great therapeutic challenge to physicians. Fortunately, in experienced highvolume centers, leak rates probably do not exceed 5% to 7% and may be as low as 1% or less [2,6,8,11]. Most leaks (75% to 85%) tend to occur at the upper sleeve portion, near the GEJ [2,8,9], for reasons that are not completely understood. Possible explanations may include decreased blood-supply to this portion of the sleeve hampering healing, thinner wall, or decreased surgical access and visualization before stapling because of the anatomical structure. Additional risk factors for leak development include inadequate sleeve-sizing, the presence of a stricture, local infection or ischemia, and performing the operation secondarily, following a previous bariatric procedure [1,2,4,5,10].
Low sleeve compliance and high luminal pressure (e.g. because of a distal stricture) were suggested as the cause of these upper leaks [10]. Importantly, bougie placement during surgery should be performed for sleeve sizing, thereby avoiding excessive or inadequate restriction [1,4]. Although bougie size negatively correlates with leak rates, too generous sleeve-sizing may result in inadequate weight loss following surgery. Other local risk factors include infection and ischemia. It was suggested that staple-line ischemia may result following dissection of the greater curvature during surgery, and not merely due to stapling itself [8], indicating that staple-line ischemia to some extent is probably an inevitable consequence in LSG. Interestingly, complication rates may also be higher when LSG is performed secondarily, after a previous bariatric intervention [2,5], potentially due to anatomical changes and more difficult surgical access. Leaks are generally categorized by the timing of their appearance [1,6] as acute (within 7 days), early (1-6 weeks), late (after 6 weeks) and chronic (after 12 weeks). It is traditionally regarded that stapling failure presents as an acute leak within the first few days, while ischemic fistulas present later in the course of healing, 5 days or more after surgery [12]. The latter are probably the most common [2,13], questioning the significant of intra-operative leak-testing [2]. The clinical significance of leaks may vary greatly, ranging from small perforations and micro-abscesses to massive uncontrolled leaks, with peritonitis and profound sepsis and shock.
Because leaks are of such a concern, many surgeons reinforce the staple-line with either additional stitching or application of biocompatible materials, which may reduce leak rates [14,15]. Nevertheless, there is no current evidence to unequivocally support buttressing or suture-supporting the staple-line [2,4]. Additional techniques to reduce leak rates include withholding tissue pressure for a short while before initiating stapling to allow tissue fluids drainage [12,16], and leaving the nasogastric tube for 24 hours after surgery to allow continuous sleeve decompression [8].
If not diagnosed promptly, leaks may either form chronic fistulas, or result in life threatening complications. Moreover, although mortality risk following LSG is very low, a leak may substantially increase it [2,17].
Clinical findings that may indicate a leak following LSG include abnormal drainage, the occurrence of abdominal pain (possibly radiating to the left scapula), tachycardia, fever, and vomiting.
Leak diagnosis is usually established performing a CT scan, swallow tests, endoscopy, or methylene-blue ingestion. Drain amylase levels may also be evaluated [18]. Importantly, high index of clinical suspicion is paramount for diagnosis.
Treatment of post-LSG leaks and fistulas may be challenging, and multiple approaches are applied in this setting.
Hemodynamically unstable patients with either contained or non-contained leaks should be re-operated as well as hemodynamically stable patients with non-contained leaks [1,2].
Operation in such instances includes multiple and thorough peritoneal washes, drainage, and possibly suturing the leak site. Gastrostomy or feeding jejunostomy tubes may also be placed [2]. When the patient is stabilized and the leak is controlled, further treatment may include each of the following approaches, which may be utilized primarily in hemodynamically stable patients with contained leaks i.e., 1) Conservative treatment including nothing per-os (NPO), antibiotics, and possibly TPN with expectant management until spontaneous closure of the fistula occurs; 2) Stent placement allowing sleeve decompression and fistula-closure; 3) Endoscopic closure procedures utilizing clips, suturing or biocompatible glues; 4) Endoscopic intra-luminal drainage; 5) Placement of a T-tube gastrostomy; and finally, 6) Reconstitution into a RYGB or performing another surgical interventions [7,13,19-22]. The last option is relevant especially in chronic leaks where other treatments failed. Although standardization efforts are being made [23,24], including the recent elegant report by Nedelcu et. al. [9], there is still no well-established guidelines for the treatment of post LSG leaks. Furthermore, because most leaks are located near the GEJ, even less data is available regarding the treatment of lower leaks or staple-line dehiscence following LSG.
Stent placement is utilized by many surgeons, but fail rates may be quite high, and multiple side effects such as misplacement, migration, decubitus, and patient intolerance are of great concern [9,13]. Endoscopic clipping, suturing, and drainage may be limited by defect size [9].
We recently introduced a novel approach for leak repair following bariatric surgery utilizing a multidisciplinary approach to perform defect closure with fibrin glue injection (yet unpublished data. The technical procedure was described above). Briefly, a multidisciplinary team including bariatric surgeons, gastroenterologists and interventional radiologists initially performs a thorough assessment of the gastric sleeve with concomitant endoscopy and fluoroscopy via a drain-port adjacent to the leak. A decision is then made whether the defects are amenable for adhesion, and if so, fibrin glue is injected via the drain port penetrating into the sleeve. Catheter placement and sealant injection are performed during constant fluoroscopy and concomitant endoscopy. The great advantage of this approach as compared with endoscopic glue injection is glue coverage of the outer portion of the leak site, before penetrating into the sleeve.
Moon et al. recently described the clinical outcome in 539 patients after LSG [6]. In this report, leak rates were 2.8%, the majority of which were treated conservatively (NPO, intravenous antibiotics, TPN, and potential drainage under CT guidance). Leaks in 60% of the patients did not heal following a single intervention, and required 1-7 (mean 2.3) interventions for complete resolution [6].
Conclusion
In light of the present case, we suggest that a conservative approach may be suitable for treating not only single-site leaks, but even complex leaks and staple-line dehiscence following LSG, as long as the fistula is drained and controlled. Moreover, the overall amount of interventions in the current case (total of 7 endoscopies, 5 of which were therapeutic) was not unusual compared with the reports concerning more simple leaks [6,22]. To the best of our knowledge, this is the first report presenting a conservative approach using endoscopic Evicel® fibrin glue for treating multiple staple-line leaks and dehiscence following LSG. Further research is warranted in this field to establish whether indeed patience, expectant management and repeated endoscopic interventions may eventually result in salvage of a severelyleaking sleeve from further surgical interventions such as reconstitution into a RYGB.
References
- Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, et al. (2012) International sleeve gastrectomy expert panel consensus statement: Best practice guidelines based on experience of >12,000 cases. SurgObesRelat Dis 8: 8-19.
- Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, et al. (2013) Gastric leaks after sleeve gastrectomy: A multicenter experience with 2,834 patients. SurgEndosc 27: 240-245.
- Noel P, Iannelli A, Sejor E, Schneck AS, Gugenheim J (2013) Laparoscopic sleeve gastrectomy: How I do it. SurgLaparoscEndoscPercutan Tech 23: e14-16.
- Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, et al. (2013) Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg 257: 231-237.
- Guetta O, Ovnat A, Shaked G, Czeiger D, Sebbag G (2015) Analysis of morbidity data of 308 cases of laparoscopic sleeve gastrectomy-The Soroka Experience. ObesSurg 25: 2100-2105.
- Moon RC, Shah N, Teixeira AF, Jawad MA (2015) Management of staple line leaks following sleeve gastrectomy. SurgObesRelat Dis 11: 54-59.
- Court I, Wilson A, Benotti P, Szomstein S, Rosenthal RJ (2010) T-tube gastrostomy as a novel approach for distal staple line disruption after sleeve gastrectomy for morbid obesity: case report and review of the literature. ObesSurg 20: 519-522.
- Marquez MF, Ayza MF, Lozano RB, Morales Mdel M, Diez JM, et al. (2010) Gastric leak after laparoscopic sleeve gastrectomy. ObesSurg 20: 1306-1311.
- Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M (2015) Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. ObesSurg 25: 559-563.
- Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, et al. (2008) Laparoscopic sleeve gastrectomy--Volume and pressure assessment. ObesSurg 18:1083-1088.
- Aurora AR, Khaitan L, Saber AA (2012) Sleeve gastrectomy and the risk of leak: A systematic analysis of 4,888 patients. SurgEndosc 26: 1509-1515.
- Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, et al. (2004) The science of stapling and leaks. ObesSurg 14: 1290-1298.
- Quezada N, Maiz C, Daroch D, Funke R, Sharp A, et al. (2015) Effect of early use of covered self-expandable endoscopic stent on the treatment of post-operative stapler line leaks. ObesSurg 25: 1816-1821.
- Sapala JA, Wood MH, Schuhknecht MP (2004) Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: Report on 738 gastric bypass patients. ObesSurg 14: 35-42.
- Liu CD, Glantz GJ, Livingston EH (2003) Fibrin glue as a sealant for high-risk anastomosis in surgery for morbid obesity. ObesSurg 13: 45-48.
- Armstrong J, O'Malley SP (2010) Outcomes of sleeve gastrectomy for morbid obesity: a safe and effective procedure? Int J Surg 8: 69-71.
- Clinical Issues Committee of American Society for Metabolic and Bariatric Surgery (2007) Sleeve gastrectomy as a bariatric procedure. SurgObesRelat Dis 3: 573-576.
- Maher JW, Bakhos W, Nahmias N, Wolfe LG, Meador JG, et al. (2009) Drain amylase levels are an adjunct in detection of gastrojejunostomy leaks after Roux-en-Y gastric bypass. J Am CollSurg 208: 881-884.
- Baltasar A, Serra C, Perez N, Bou R, Bengochea M, et al. (2005) Laparoscopic sleeve gastrectomy: A multi-purpose bariatric operation. ObesSurg 15: 1124-1128.
- Martin-Malagon A, Rodriguez-Ballester L, Arteaga-Gonzalez I (2011) Total gastrectomy for failed treatment with endotherapy of chronic gastrocutaneous fistula after sleeve gastrectomy. SurgObesRelat Dis 7: 240-242.
- Barreca M, Nagliati C, Jain VK, Whitelaw DE (2015) Combined endoscopic-laparoscopic T-tube insertion for the treatment of staple-line leak after sleeve gastrectomy: A simple and effective therapeutic option. SurgObesRelat Dis 11: 479-482.
- Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, et al. (2015) Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID). ObesSurg 25: 1293-1301.
- Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS (2010) Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. ObesSurg 20: 403-409.
- Nedelcu M, Skalli M, Delhom E, Fabre JM, Nocca D (2013) New CT scan classification of leak after sleeve gastrectomy. ObesSurg 23: 1341-133.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences