A 6-Year Follow-Up Study on a Child with Large Vestibular Aqueduct Syndrome
Yan-Li Wang#, Bai-Cheng Xua#, Xiao-Yun Zhao and Yu-Fen Guo*
1Department of Otolaryngology-Head and Neck Surgery, Lanzhou University Second Hospital, No. 82 Cuiyingmen, Lanzhou 730030, Gansu Province, P.R. China
2Health Commission of Gansu Province, No. 220 Baiyin Road, Lanzhou 730000, Gansu Province, P.R. China
#The first two authors contributed equally to this work.
- Corresponding Author:
- Yu-Fen Guo, MD, PhD
Professor, Chief Physician
Department of Otolaryngology-Head and Neck Surgery
Lanzhou University Second Hospital, Lanzhou 730030
and Health Commission of Gansu Province
Lanzhou 730000, P.R. China
Tel: +86 931 8942468
E-mail: guoyflz@163.com
Received date: July 21, 2020; Accepted date: August 13, 2020; Published date: August 20, 2020
Citation: Wang YL, Xua BC, Xhao XY, Guo YF (2020) A 6-Year Follow-Up Study on a Child with Large Vestibular Aqueduct Syndrome. Med Case Rep Vol.6 No.4:151. DOI: 10.36648/2471-8041.6.4.151.
Abstract
Large vestibular aqueduct syndrome (LVAS) is considered to be the most frequent morphogenetic cause of hearing loss in children, however, conservative treatments can't ultimately cure hearing loss caused by LVAS, and cochlear implantation has proved to be one of the best options to restore the hearing of patients with severe-to-profound deafness. In this paper, we followed up a child with LVAS closely, with frequent audiometric evaluations and health examination for 6 years; he experienced hearing loss from moderate to profound. Finally, he received a left cochlear implant. After a period of postoperative language rehabilitation training, the patient's residual hearing, especially in the low frequency, has not disappeared but has been retained at 18 months and 30 months after surgery. Now, he gets along well with their classmates in school and adapts well to social activities. We suggest that patients exhibiting LVAS should receive a CI at an earlier stage in hearing loss, when they have better speech perception, so as to established excellent speech perception outcomes.
Keywords
Large vestibular aqueduct syndrome; Cochlear implant; Residual hearing
Introduction
Large vestibular aqueduct syndrome (LVAS) is considered to be the most frequent morphogenetic cause of hearing loss in children [1], and it is often associated with other congenital inner ear anomalies, the most common being an abnormally large vestibule, an enlarged semicircular canal, or a hypoplastic cochlea [2]. The clinical features of LVAS have been described to be fluctuant and progressive hearing loss [3,4] vertical and axial width larger than 1.5 mm on the midpoint between labyrinth and operculum at temporal bone highresolution computed tomography (HRCT) [5]. Walsh et al. [6] reported that sensorineural hearing loss (SNHL) in patients affected by LVAS were fluctuant and progressive, often with sudden, stepwise onset or progression, secondary to trigger activities such as those involving the Valsalva maneuver, minor head trauma, scuba diving, jogging, common colds, and so forth.
In this paper, we observe a child with bilateral enlarged vestibular aqueduct and Mondini deformity for 6 years, the degree of his hearing loss ranged from moderate initially to profound finally. For the past three years, his hearing has suddenly worsened after a cold or a minor head trauma, so that he had to accept a cochlear implant at last. With the help of a cochlear implant, he can communicate normally with his peers now.
Case Presentation
In this paper, we reported a 10-year-old hearing impaired boy with bilateral enlarged vestibular aqueduct and Mondini deformity. Speech development was normal until the age of 4 when his parents first suspected disease because their son’s pronunciation was not clear, they took the child to the Lanzhou University Second Hospital for a correlative check-up and an audiological evaluation.
When the patient was firstly checked audiological evaluation in 2014, ABR showed the threshold of right ear was 100 dBnHL, while the left ear was 80 dBnHL when motivated at 100 dBnHL. Distortion products otoacoustic emissions (DPOAEs) were absent at all frequencies(0.5 KHz-8 KHz) bilaterally. ASSR indicated that the thresholds of left ear at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz frequencies were 90 dB HL, 80 dB HL, 90 dB HL and 100 dB HL, respectively, while the thresholds of right ear were all 100 dB HL at the above four frequencies.
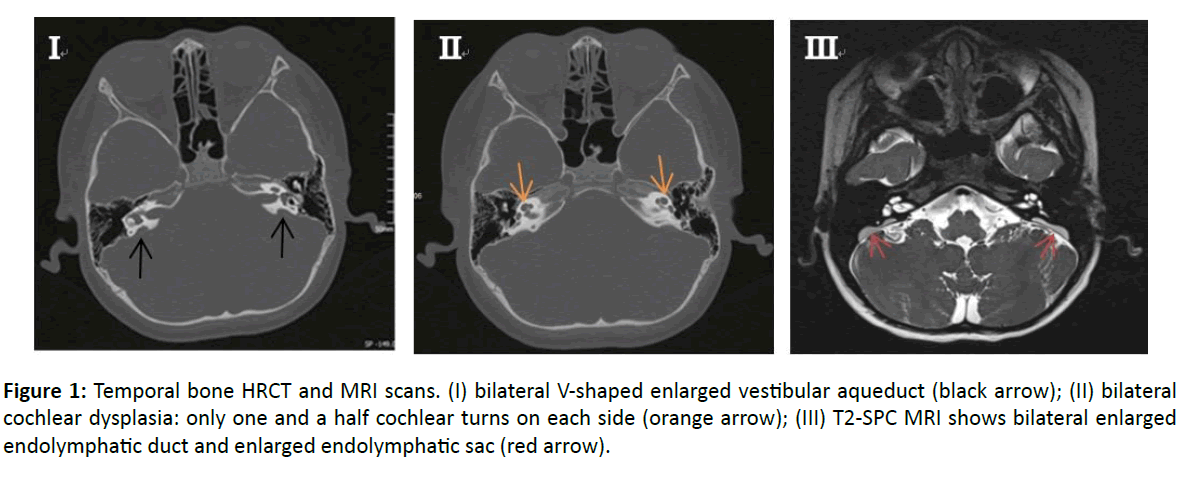
HRCT and MRI scans of the inner ear showed bilateral cochlear dysplasia as follows: one and a half cochlear turns instead of the classical 2.5, with the apex showing a V-shaped enlarged vestibular aqueduct bilaterally (Figure 1). In axial T2- weighted MRI images an enlarged endolymphatic duct and enlarged endolymphatic sac bilaterally. DNA was extracted from collected samples of peripheral blood. All of the 21 exons of SLC26A4 including the flanking sequences were amplified by the polymerase chain reaction (PCR) using genomic DNA [7].
Figure 1: Temporal bone HRCT and MRI scans. (I) bilateral V-shaped enlarged vestibular aqueduct (black arrow); (II) bilateral cochlear dysplasia: only one and a half cochlear turns on each side (orange arrow); (III) T2-SPC MRI shows bilateral enlarged endolymphatic duct and enlarged endolymphatic sac (red arrow).
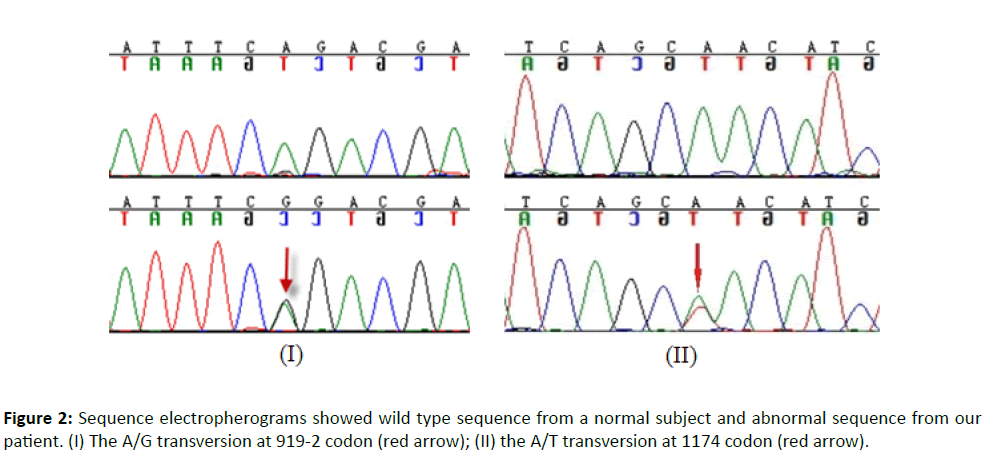
The genotyping and sequencing were conducted at The Beijing Genomics Institute (Shenzhen). The molecular analysis of the SLC26A4 gene showed a compound heterozygote mutation of c.919-2A>G and c.1174A>T (Figure 2) in the patient, these two mutation sites have been confirmed pathogenic mutations [8].
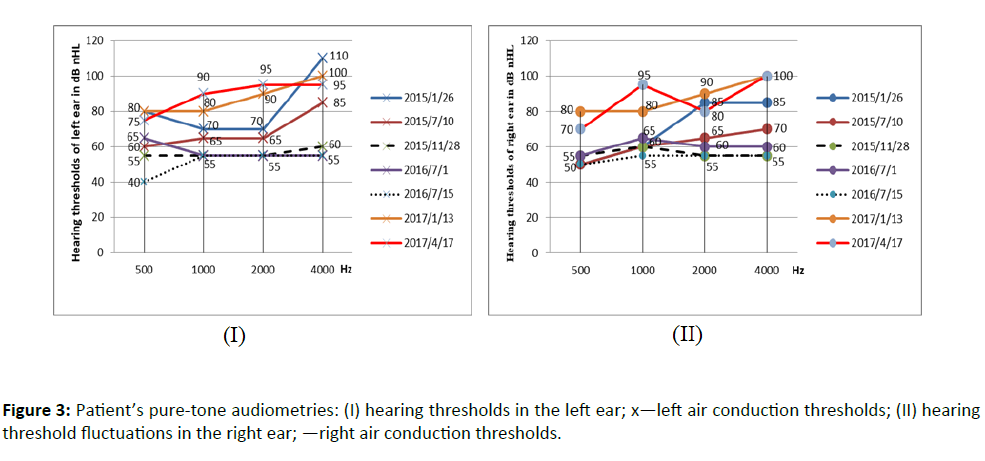
After those tests, he was diagnosed with sensorineural hearing loss affected by LVAS. He then wore hearing aids in both ears. In the following three years, his hearing (as shown in Fig.3, 2015/7/10 and 2016/7/1) suddenly decreased after catching a cold or something hitting his head, but after timely nutritional neurological drugs treatment such as Ginaton and small dose of glucocorticoids such as dexamethasone, his hearing (as shown in Figure 3, 2015/11/28 and 2016/7/15) improved.
However, his hearing eventually became profound hearing loss, and hearing aids could not make up for his hearing loss. So he received a left cochlear implant (Med-el SONATA, Austria) under general anesthesia at the age of eight (October 2017).
The cochlear implant adjustment and postoperative language rehabilitation training were successfully started one month after CI, and the right ear was wearing a hearing aid. He received hearing and speech assessment at 1, 6, 12, 18 and 30 months after CI with the Category of Auditory Performance (CAP) and Speech Intelligibility Rating (SIR), and at 12, 18 and 30 months after CI with the Computer-aided Chinese Speech Auditory Platform, which includes recognition rate of monosyllabic word in quiet, spondaic word in quiet and sentence in quiet and at +10dB and +5dB signal-to-noise ratios (SNRs). The CAP scores at 1 month, 12 months, 18 months and 30 months after surgery were 4, 6, 6 and 7 respectively, while SIR scores in all four periods were 4.
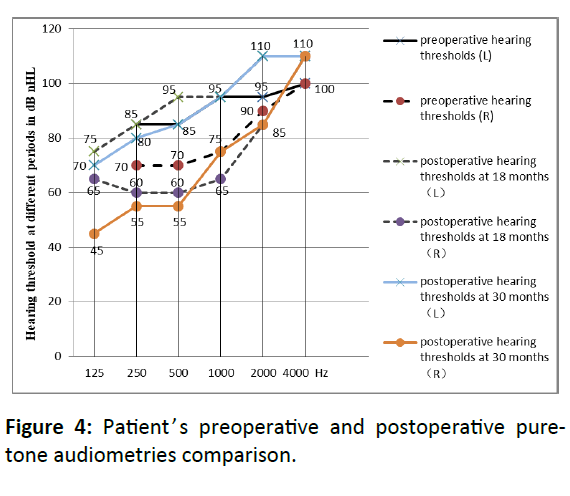
The speech recognition rate showed a constantly advancement after surgery. At 12 months after CI, the recognition rates of monosyllabic word, spondaic word and sentence in quiet were 66.67%, 63% and 72% respectively, and sentence at +10dB SNR was 58%. At 18 months after CI, the recognition rates of monosyllabic word, spondaic word and sentence in quiet were 82.67%, 77%, and 86%, and sentence at +10 and +5dB SNRs were 84% and 58%. And then 30 months after CI, the recognition rates of monosyllabic word, spondaic word and sentence in quiet were 84%, 82%, and 88%, and sentence at +10 and +5dB SNRs were 86% and 62%, which were significantly improved. Preoperative and postoperative hearing thresholds at 18 months and 30 months were shown in Figure 4.
Discussion
In this paper, we followed up a child with large vestibular aqueduct syndrome closely, with frequent audiometric evaluations and health examination for 6 years. Hearing impairment by LVAS is characterized by fluctuating SNHL, especially in the high tone. Bilateral moderate to profound hearing loss occurs in early childhood [9]. The patient in this paper was not diagnosed with hearing loss until he was 4 years old. As depicted in Fig.3, we can see that the general trend of hearing change is a fluctuation and decline gradually, and finally stable to profound hearing loss. The patient experienced hearing loss from moderate to profound over a period of 3 years, appropriate medication can delay the decline of hearing loss.
However, conservative treatments such as nutritional neurological drugs can't ultimately cure hearing loss caused by LVAS, cochlear implantation has proved to be one of the best options to restore the hearing of patients with severe-toprofound deafness and to help them return to the world of sound [10]. Cochlear implants have been widely used for treatment of childhood deafness for more than 20 years, and they have dramatically changed the treatment and prognosis for patients with profound sensorineural hearing loss. Deaf adults and children can be successfully (re)integrated into the hearing world through a multidisciplinary approach involving otolaryngologists, audiologists, and speech/language pathologists [11]. A study has showed that individuals with LVAS and hearing loss obtain excellent outcomes with a CI [12].
Conclusion
In this paper, the patient's residual hearing, especially in the low frequency, has not disappeared but has been retained at 18 months and 30 months after surgery. In addition, the residual hearing in the right ear after surgery was significantly improved compared with preoperative at 125 Hz, 250 Hz and 500 Hz frequencies. Two-mode cochlear and hearing aid interventions can help patient listen better. Now, the patient in this paper gets along well with his classmates in school and adapts well to social activities after CI.
Conflict of Interest
The authors declare that they have no competing interests.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Yu-Fen Guo, grant number 81570926), (Yi-Ming Zhu, grant number 81960192); the Doctoral Tutor Project of Lanzhou University Second Hospital (Yu-Fen Guo, grant number 200463); Gansu Health Industry Scientific Research Project (Xiao-Wen Liu, grant number GSWSKY2017-11); the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (Yi-Ming Zhu, grant number CY2017–QN14); and the Gansu Provincial Youth Science and Technology Fund Projects (Yi-Ming Zhu, grant number 1606RJYA227).
Patient’s Consent
Informed consent was taken from the patient and his parents to publish his case as a case report.
References
- Puls T, Van Fraeyenhoven L (1997) Large vestibular aqueduct syndrome with mixed hearing loss: a case report. Acta Oto-Rhino-Laryngologica Belgica 51: 185-189.
- Emmett JR (1985) The large vestibular aqueduct syndrome. Am J Otol 6: 387-415.
- Berrettini S, Forli F, Bogazzi F, Neri E, Salvatori L, et al. (2005) Large vestibular aqueduct syndrome: Audiological, radiological, clinical, and genetic features. Am J Otolaryngol 26: 363-371.
- Tong KA, Harnsberger HR, Dahlen RT, Carey JC, Ward K (1997) Large vestibular aqueduct syndrome: A genetic disease? AJR Am J Roentgenol 168: 1097-1101.
- Sennaroglu L, Bajin MD (2017) Classification and Current Management of Inner Ear Malformations. Balkan Med J 34: 397-411.
- Walsh RM, Ayshford CA, Chavda SV, Proops DW (1999) Large vestibular aqueduct syndrome. ORL J Otorhinolaryngol Relat Spec 61: 41-44.
- Wang QJ, Zhao YL, Rao SQ, Guo YF, Yuan H, et al. (2007) A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clin Genet 72: 245-254.
- Luo J, Bai X, Zhang F, Xiao Y, Gu L, et al. (2017) Prevalence of mutations in deafness-causing genes in cochlear implanted patients with profound nonsyndromic sensorineural hearing loss in Shandong Province, China. Annals of human genetics 81: 258-266.
- Lai CC, Shiao AS (2004) Chronological changes of hearing in pediatric patients with large vestibular aqueduct syndrome. Laryngoscope 114: 832-838.
- Asma A, Anouk H, Luc VH, Brokx JP, Cila U, et al. (2010) Therapeutic approach in managing patients with large vestibular aqueduct syndrome (LVAS). Int J Pediatr Otorhinolaryngol 74: 474-481.
- Copeland BJ, Pillsbury HC (2004) 3rd. Cochlear implantation for the treatment of deafness. Annu Rev Med 55: 157-167.
- Hall AC, Kenway B, Sanli H, Birman CS (2019) Cochlear implant outcomes in large vestibular aqueduct syndrome-should we provide cochlear implants earlier? Otol Neurotol 40: e769-e773.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences