Changes in The Expression of Somatostatin (SOM) in Nerve Fibers of Gastrointestinal Mucosa in Dogs with Inflammatory Bowel Disease (IBD)
Krystyna Makowska
Krystyna Makowska*
Faculty of Veterinary Medicine, Department of Clinical Physiology, University of Warmia and Mazury in Olsztyn, Oczapowski, Olsztyn, Poland
- *Corresponding Author:
- Krystyna Makowska
Faculty of Veterinary Medicine
Department of Clinical Physiology
University of Warmia and Mazury in Olsztyn
Oczapowski Str. 13, 10-718 Olsztyn, Poland
Tel: +48 (89) 523 49 13
Fax: +48 (89) 524 04 08
E-mail: krystyna.makowska@uwm.edu.pl
Received Date: January 25, 2019; Accepted Date: February 12, 2019; Published Date: February 14, 2019
Citation: Makowska K (2019) Changes in The Expression of Somatostatin (SOM) In Nerve Fibers of Gastrointestinal Mucosa in Dogs with Inflammatory Bowel Disease (IBD). Med Case Rep Vol.5 No.1:90.
Abstract
Aim and Background: The aim of this study was to demonstrate for the first time changes in the number of somatostatin – like immunoreactive (SOM-LI) nerve fibers within the mucosal layer of the canine intestine during inflammatory bowel disease (IBD). Mostly, somatostatin (SOM) causes relaxation of the intestinal smooth muscles, as well as reduces the secretion of digestive enzymes and a wide range of gut hormones, such as gastrin, vasoactive intestinal polypeptide and cholecystokinin. Moreover, in the light of the previous studies it is known that SOM participates in mechanisms connected with various intestinal pathological processes, but the knowledge on this issue is rather fragmentary.
Results and Discussion: The study was performed on mucosal biopsy specimens collected from the duodenum, jejunum and descending colon of healthy dogs and dogs with varied severity of IBD. The density of these nerves immunoreactive to SOM was determined by single immunofluorescence technique. The obtained results indicate that IBD induces changes in the density of SOM-LI nerve fibers in canine enteric mucosal layer, and the character of these changes depend on the fragment of the intestine and severity of disease process. SOM-positive fibers density was higher in dogs with severe IBD than in other group of animals, and the most visible changes have been noted in the jejunum.
Conclusion: The obtained results suggest that SOM in intestinal nerve fibers could play a role in the pathogenesis and development of canine IBD. SOM probably shows activity protecting the intestine against damaging factors accompanying IBD. It can be expected that observed changes result from adaptive, anti-inflammatory and anti-nociceptive properties of this peptide. Whereas probably the key role in mechanisms connected with IBD plays the increase in the sensitivity within gut-associated lymphoid tissue (GALT), which leads to disorders in the intestinal activity. This observation may the first step to establish the treatment of canine IBD with somatostatin analogs. We report a first case of chemical pneumonitis secondary to aspiration associated with a patient that had gastric balloon placed, despite following normal NPO guidelines. Standard ASA guidelines may not be sufficient for patients with gastric balloons undergoing elective surgery. These patients may pose a greater risk of aspiration while undergoing emergency procedures.
Keywords
Digestive tract; Dogs; Enteric nervous system; Immunohistochemistry; Somatostatin
Abbreviations
SOM-LI: Somatostatin–Like Immunoreactive; SOM: Somatostatin; IBD: Inflammatory Bowel Disease; GALT: Gut-Associated Lymphoid Tissue; NHE8: Sodium/Hydrogen Exchanger 8; ENS: Enteric Nervous System; Group I: Group with Mild IBD; Group II: Group with Moderate IBD; Group III: Group With Severe IBD; GI Tract: Gastrointestinal Tract; SEM: Standard Error of the Mean
Introduction
Somatostatin (SOM), was first discovered within the ovine hypothalamus in 1973 [1], is a peptide, which has two active forms: one of 14 amino acids and the other of 28 amino acids and may act on six types of specific receptors SSTR1, SSTR2A, SSTR2B, SSTR3, SSTR4 and SSTR5, all of which are members of the G protein coupled receptor superfamily [1-3]. It is known that SOM occurs in the various parts of the central and peripheral nervous system, where it plays multidirectional functions [3,5-9]. This peptide has been also described in neurons innervating the gastrointestinal tract of various mammals’ species, including human [2,8,10-14], where amongst other dozens of neuronal factors, plays role of neuromediator and/or neuromodulator. SOM is present both within the enteric nervous system (ENS) located in the wall of the stomach and intestine [2,12,13], as well as in the extrinsic innervation of the digestive tract, which is composed of neurons situated in sympathetic, parasympathetic and sensory ganglia [6,9,15]. The number of enteric neurons, which show the presence of SOM clearly depend on mammal’s species studied, fragments of the GI tract and the types of the enteric ganglia [2,8,11,12,16]. These differences in the expression of SOM indicate that the roles of this peptide also depend on the fragment of the digestive tract, but it should be underlined that the exact roles of SOM within the neuronal structures supplying the GI tract still remain not fully explained. In the light of the previous studies it is known that that first of all this peptide possesses inhibitory activity. Namely, SOM causes relaxation of the intestinal smooth muscles, as well as reduces the secretion of digestive enzymes and a wide range of gut hormones, such as gastrin, vasoactive intestinal polypeptide and cholecystokinin [2,3,17]. Moreover, the experimental administration of the described peptide resulted in the decrease of the intestinal blood flow, capillary surface area and oxygen consumption [18]. Especially the knowledge concerning the functions of SOM during intestinal pathological processes is extremely limited. Previous investigations have described that neurochemical characterization of the nervous structures supplying the GI tract may be modified under various pathological conditions, and these changes also concern the expression of somatostatin. Nevertheless, although it is known that SOM can affect the immunological system and shows anti-inflammatory activity [2,3,19,32], the exact mechanisms underpinning the fluctuation of SOM expression in the intestinal nerves are unknown. Moreover, previous studies have shown fluctuations in the expression of SOM in the intestinal nervous structures under experimental pathological processes caused by factors applied into the lumen or wall of the intestine, whereas data on the impact of spontaneous diseases affecting the GI tract on somatostatin – like immunoreactive (SOM-LI) intestinal nervous structures and the role of this peptide in mechanisms associated with such diseases are limited [2,3,19,20].
Therefore, the aim of this study was to investigate the changes in immunoreactivity to SOM in the nerves supplying the mucosal layer of the GI tract under inflammatory bowel diseases in dogs. It should be underlined that IBD is actually the group of protracted disorders of the GI tract with an undetermined etiology [20,21]. In the light of previous investigation, it seems to be that the key role in mechanisms connected with IBD plays the increase in the sensitivity within gut-associated lymphoid tissue, which leads to disorders in the intestinal activity [22-24]. It is also known that there are a lot of factors favoring the progression of IBD, involving genetic predisposition, stress, environmental contamination with toxic substances, parasites, food allergies, side effects of drugs and many others [20,23-25]. IBD in dogs is accompanied by a wide range of non-specific symptoms, including among others loss of appetite, weight decreased, vomiting and recurrent diarrhea [19-21,23]. Due to an unidentified etiology and non-specific symptoms, which first of all consists in exclude other intestinal disorders, diagnosis and effective treatment of IBD in dogs are extremely difficult [20,21,23]. In recent years the attention is drawn to the roles of neuronal active substances taking part in the intestinal innervation in mechanisms associated with IBD. It is known that the expression of some neuromediators and/or neuromodulators may be subject to modification in this disease, what suggest active participation of these substances in intestinal adaptive processes during IBD [19-21,24]. It should be underlined that SOM, which has been reported as an relatively well-known anti-inflammatory and anti-nociceptive factor [17], can play an important role in described diseases, but till now the influence of IBD on somatostatin – like immunoreactive (SOM-LI) nerves in the canine GI tract has not been studied at all.
Research Methodology
This study was made up on 28 German shepherd hybrid dogs of both sexes with body weight of 15 to 25 kg, aged 6 to 10 years, divided into 4 groups (7 animals in each). The control group consisted of healthy dogs were included into the investigation based on the results of IBD screening tests conducted in a dog shelter in Olsztyn (Poland). The experimental groups were formed out from patients of the Veterinary Clinic of the University of Warmia and Mazury in Olsztyn. The studies were performed according to suggestions of the Local Ethics Committee for Animal Experimentation in Olsztyn (Decision No. 47/2009/DTN). Animals to the particular groups were qualified based on the results of clinical, laboratory, endoscopic and histopathological examinations of sections of duodenal, jejunal and colonic mucosa.
Dogs with a suspicion of IBD were undergone the biochemical, radiological, parasitological, bacteriological and mycological stool tests, as well as provocation trials to exclude other intestinal diseases with symptoms similar to IBD. Dogs with confirmed IBD were divided into experimental groups based on CIBDAI scores [26] and histopathological changes [27]. These groups were as follows:
• Group I – Mild IBD, CIBDAI score – 4-5 points, histopathological score "+";
• Group II – Moderate IBD, CIBDAI score – 6-8 points, histopathological score "++";
• Group III – Severe IBD, CIBDAI score – 10-16 points, histopathological score "+++".
Specimens were obtained from all dogs included into the investigation during gastroscopic or colonoscopy examinations with the use of FB-24U-1 biopsy forceps with a diameter of 2.5 mm and FB-50U-1 biopsy forceps with a diameter of 3.7 mm (Olympus). Immediately after collection, biopsy specimens from the duodenum, jejunum and descending colon (three from the particular fragment of the GI) were fixed in 4% buffered paraformaldehyde solution for 15 minutes. Then they were rinsed in phosphate solution (pH 7.4) for three days, transferred to 18% buffered sucrose solution and stored at 4°C. After at least three weeks fragments of the intestine were frozen in -20°C, cut on 10 micrometer sections using the Microm cryostat (HM525, Walldorf, Germany) and placed on gelatin coated slides.
Tissues were subjected to single immunofluorescence technique according the method of Gonkowski [8]. In short, intestinal specimens were dried for 45 minutes at room temperature (rt) and incubated with blocking solution containing 10% goat serum, 0.1% bovine serum albumin (BSA), 0.01% NaN3, Triton X-100 and thimerosal in PBS for 1 hour (rt). Then tissue samples were incubated overnight in a humidity chamber (rt) with anti-SOM antibody (rat monoclonal, Biogenesis Ltd., Poole, UK, working dilution 1:100). The next day, tissues were incubated with a specific secondary antibody conjugated to Alexa Fluor 546 (Donkey, anti-rat, Invitrogen Carlsbad, CA, USA, working dilution 1:1000) for 1 h (rt). Then the specimens were treated with glycerol solution in PBS (1:2; pH 7.4) and were covered with coverslips.
Fragments of the intestine were evaluated under the Olympus BX51 fluorescence microscope equipped with appropriate filters. Mucosal fibers were counted using a semi-quantitative evaluation determinating the number of somatostatins – like immunoreactive (SOM-LI) fibers in the field of microscopic view (0.1 mm2). Fibers were counted in four fields of view in three sections of every biopsy specimen from every evaluated intestinal segment (duodenum, jejunum and colon).
In every dog included into the investigation, a total of 36 fields of view in every segment were evaluated. The evaluated fields were separated by a minimum distance of 100 μm to avoid repeated counts. The results were grouped, mean values and standard deviation were calculated. Microphotographs were captured with a digital camera in the Analysis 3.0 application. The specificity of the used primary antibody was verified by typical methods, including a pre-absorption, replacement and omission tests.
The significance of differences between groups was determined by the Kruskal-Wallis test at p≤0.05 (significant) and p≤0.01 (highly significant). The results were processed in the Statistica 9.1 application (StatSoft, Inc.).
Results
Nerve fibers immunoreactive to SOM were present in the mucosal layer of all fragments of the GI tract studies in all animals included into the experiment, but the number of such fibers were relatively scanty (Table 1).
| Variables | Group C | Group I | Group II | Group III |
|---|---|---|---|---|
| Duodenum | 1.02 ± 0.19D | 0.88 ± 0.27D | 0.92 ± 0.19 | 1.54 ± 0.40 A, B |
| Jejunum | 0.98 ± 0.28D | 0.94 ± 0.24 | 0.97 ± 0.16 | 1.57 ± 0.38A |
| Colon | 0.90 ± 0.19D | 1.06 ± 0.42 | 0.86 ± 0.11 | 1.16 ± 0.29A |
A – Significantly different from control; B – Significantly different from group I; C – Significantly different from group II; D – Significantly different from group III; Kruskal-Wallis test; p<0.05 – lowercase letters; p<0.01 – uppercase letters
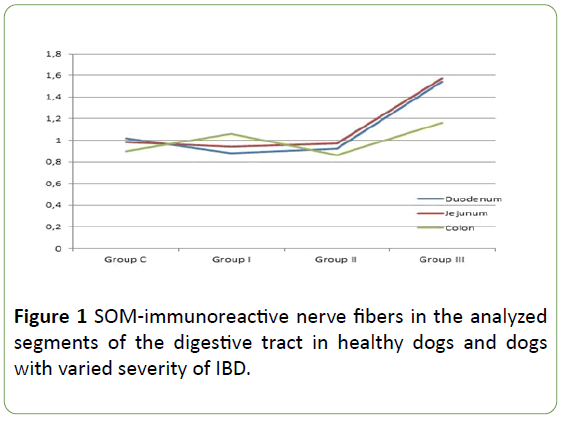
Table 1: The number of mucosal SOM-immunoreactive nerve fibers in one field of view in the duodenum, jejunum and descending colon of control group (C) dogs and dogs with mild (Group I), moderate (Group II) and severe (Group III) IBD.
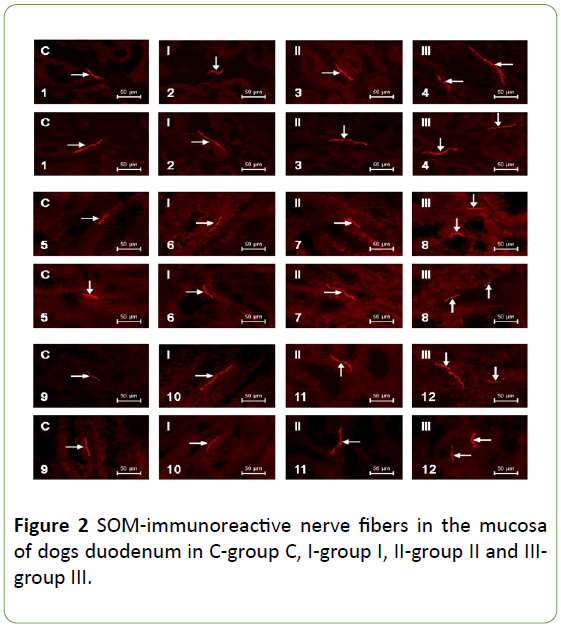
In healthy dogs the number SOM-LI nerves on average amounted to 1.02 ± 0.19 fibers per microscopic observation field in the duodenum, 0.98 ± 0.28 in the jejunum and 0.90 ± 0.19 in the descending colon. Described fibers are thin and delicate.
In animals of group 1 (patients with mild IBD) and group 2 (dogs suffering from moderate IBD) the number of SOM-positive nerves were not statistically significantly different from the values observed in control animals. The number of SOM-LI nerves in dogs with mild IBD achieved on average 0.88 ± 0.27, 0.94 ± 0.24 and 1.06 ± 0.42 fibers/observation field in the duodenum, jejunum and colon, respectively. In turn, in animals suffering from moderate IBD these values amounted to 0.92 ± 0.19 in duodenal, 0.97 ± 0.16 in jejunal and 0.86 ± 0.11 in colonic mucosal layer. Morphological characteristics of nerves, which showed the presence of SOM observed in dogs with mild and moderate IBD were the same as under physiological conditions (Table 1, Figures 1 and 2).
Contrary to mild and moderate IBD, severe form of this disease caused statistically significant increase (in the comparison to the control animals) in the number of SOM – positive mucosal layer of all fragments of the GI tract studied. The average number of such fibers per observation field in dogs with severe IBD amounted to 1.54 ± 0.40, 1.57 ± 0.38 and 1.16 ± 0.29 in the duodenum, jejunum and descending colon, respectively.
Moreover, nerves immunoreactive to SOM observed in the GI tract of dogs with severe form of IBD were different from those noted in other groups of animal. They were thick, long and good visible (Table 1, Figures 1 and 2).
Discussion
In the light of the previous studies it is known that SOM occurs in the nervous structures of the GI tract of a wide range of mammals including humans [2,19,10,14,23,25,28-34], and the number of SOM-LI enteric neurons and nerves explicitly depends on the animal species studied and the segment of the GI tract. Generally, the majority of enteric nervous structures immunoreactive to SOM has been observed within submucous plexus of the enteric nervous system located near the lamina propria of the mucosal layer and regulating the secretory activity of this part of the intestinal wall [2,14,23,25,34]. In turn, the population of SOM-LI neurons in the myenteric plexus of the enteric nervous system placed between the longitudinal and circular muscle layer of the intestinal wall is rather limited, and SOM has been observed in efferent cholinergic neuronal cells [2,14,23]. Contrary to the previous investigations concerning the other animal species [2,23,25,35-40], the population of SOM-positive nerves noted in the canine GI tract during the present study is extremely limited. These interspecies differences in distribution and number of SOM-LI nervous structures strongly suggest that described peptide play different roles depending on the animal species. It should be underlined that the exact functions of SOM located in the nervous system supplying the GI tract is not fully elucidated. In the light of the previous studies SOM within the intestine is known as an inhibitory factor, which shows relaxant effects on the smooth muscles, reduces secretion of enzymes, hormones and other neuromediators, as well as dilates the blood vessels within the intestinal wall and mesentery [2,19,21,35-40].
Apart the physiological conditions, SOM plays some important roles during intestinal pathological states. First of all this peptide is considered to be one of the main factors taking part in anti-inflammatory processes. It has been shown that SOM by the direct interaction with receptors on the intestinal epithelial cells inhibits the secretion of pro-inflammatory factors and therefore participates in the response of the intestinal mucosal layer to inflammatory processes [3,19,38]. In turn, investigations on the rodents with experimentally-induced inflammatory processes has shown that SOM may modulate the intensity of the inflammation by interference in the interactions between neuronal and mast cells, which indicates that inflammatory process activates not only pro-inflammatory cytokines synthesis, but also endogenous system preventing the tissue damage [33]. Other functions of SOM during intestinal pathological processes are connected with reduction of pain stimuli conduction and regulatory processes within the immunological system [5,2,19]. SOM during the intestinal inflammation may also play protective functions preventing the mucosal injury. Namely, it is known that this peptide enhances the synthesis of sodium/hydrogen exchanger 8 (NHE8) – the factor responsible to water/sodium absorption in the intestine, loss of which during inflammation results in mucosal injury [17].
The results obtained during the present study have shown that canine IBD induces changes in the number of SOM-LI nerves in the mucosal layer of various parts of the GI tract, but these changes are visible only in severe form of the disease. This fact may indicate that above mentioned mechanisms connected with the participation of SOM in adaptive and protective processes are put into effect only under the advanced stage of inflammatory processes. Such situation may be also connected with the fact that symptoms of pain in the canine IBD are the most intensive in severe form of this disease, and the increase of SOM-positive nerves relates to the inhibitory influence of this peptide on the pain stimuli conduction, which is realized by activation SSt4 receptor subtype [5]. The present results confirm the previous observation on other mammals’ species. Namely, it is known that he expression of SOM in the intestinal innervation may undergo changes during ulcerative colitis and Crohn disease in human [32,39], as well as in experimental – induced inflammation and nerve damage in the domestic pig [9]. However, the exact mechanisms leading to the increase in the number of intestinal SOM-LI nerves in the canine IBD still remain unknown. They may be caused by the changes in different stages of peptide synthesis, including transcription and/or translation, as well as fluctuations in the transport of SOM from neuronal cell bodies to the nerve endings.
Nevertheless, obtained results, which clearly show that SOM participates in pathological processes during severe IBD may be the first step to introduce in the future new treatments of canine IBD with somatostatin analogs. It is relatively well known that one of SOM analogs – octreotide is used in medicine in therapy of growth hormone producing tumors and acromegaly [4,15,18]. It may be also suitable for the treatment of diseases concerning the digestive tract, including diarrhea and flushing accompanying the carcinoid, persistent diarrhea in AIDS and Verner–Morrison syndrome and acute hemorrhage from esophageal varices in liver cirrhosis [6,30,31]. Octreotide is also used in veterinary medicine during therapy of tumors in dogs and cats. Although it has been shown that octreotide, apart from antidiarrheal activity, effectively decreases mucosal damage in experimental colitis [7], reduces the magnitude inflammatory changes and improves intestinal absorption of nutrients during inflammation [18,35-39], till now this somatostatin analog has not been used in the canine IBD. Results of this study, presenting functions of SOM in mechanisms connected with IBD in dogs, are the prerequisite for initiation of investigations aimed at the establishing of IBD therapy based on analogs of this peptide [40].
Conclusion
the results obtained during the present study have demonstrated that severe form of IBD results in the increase in the number of SOM-LI intramucosal nerves in the duodenum, jejunum and descending colon of the dog. These observations suggest the participation of SOM in the mechanisms of pathological processes connected with this disease. Due to the fact that etiology of canine IBD is very complex and not fully elucidated, exact mechanisms of observed changes remain unknown. Nevertheless, in the light of the previous studies concerning roles of SOM in various intestinal pathological processes in other species, it can be expected that observed changes result from adaptive, anti-inflammatory and antinociceptive properties of this peptide. So, SOM probably shows activity protecting the intestine against damaging factors accompanying IBD. This observation may the first step to establish the treatment of canine IBD with somatostatin analogs. However, further researches on such possibilities are needed.
Funding
This study was supported by the National Center for Research and Development under grant No. N N308 234938 and by KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal - Safe Food” (Ministry of Science and Higher Education, Decision No. 05-1/KNOW2/2015).
Authors’ Contributions
AR is the originator and main performer of experiment. SG is the major contributor in writing the manuscript. KM takes part in the statistical analysis of data creation of figures. EK collected tissues for research. JC checked the manuscript linguistically and grammatically. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study was performed according to suggestions of the Local Ethics Committee for Animal Experimentation in Olsztyn, Decision No. 47/2009/DTN.
References
- Allenspach K, Wieland B, Gröne A, Gaschen F (2007) Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 21:700-708.
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, et al. (1973) Hypothalamic polypeptide that inhibits the secretion of immunoreactive growth hormone. Science 179: 77-79.
- Chowers Y, Cahalon L, Lahav M (2000) Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol 165: 2955-2961.
- Cuevas-Ramos D, Fleseriu M (2016) Pasireotide: A novel treatment for patients with acromegaly. Drug Des Devel Ther 10: 227-239.
- Dahaba AA, Mueller G, Mattiassich G, Rumpold-Settinger G, Bornemann H, et al. (2009) Effect of somatostatin analogue octreotide on pain relief after major abdominal surgery. Eur J Pain 8: 861-864.
- Deng C, Deng B, Jia L, Tan H (2017) Efficacy of long-acting release octreotide for preventing chemotherapy-induced diarrhoea: protocol for a systematic review. BMJ Open 7: e014916.
- Eliakim R, Karmeli F, Okon E, Rachmilewitz D (1993) Octreotide effectively decreases mucosal damage in experimental colitis. Gut 34: 264-269.
- Gonkowski S (2013) Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 39: 542-551.
- Gonkowski S, Calka J (2010) Changes in the somatostatin (SOM)-like immunoreactivity within nervous structures of the porcine descending colon under various pathological factors. Exp Mol Pathol 88: 416-423.
- Gonkowski S, Rychlik A, Calka J (2013) Somatostatin as an active substance of enteroendocrine cells in the canine digestive tract in physiological conditions and during inflammatory bowel disease. Eur J Inflamm 1: 655-661.
- Gonkowski S, Rychlik A, Nowicki M, Nieradka R, Bulc M, et al. (2012) A population of nesfatin 1-like immunoreactive (LI) cells in the mucosal layer of the canine digestive tract. Res Vet Sci 93: 1119-1121.
- Jana B, Calka J, Rytel L, Czarzasta J (2015) Morphological and neurochemical characterization of the ovarian sympathetic chain ganglia perikarya in testosterone-treated sexually matured pigs. Ann Anat 202: 28-35.
- Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, et al. (2003) A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 17:291-297.
- Keast JR, Furness JB, Costa M (1984) Somatostatin in human enteric nerves. Distribution and characterization. Cell Tissue Res 237: 299-308.
- Kumar Das R, Banerjee S, Shapiro BH (2013) Noncanonical suppression of growth hormone – dependent isoforms of cytochrome P450 by the somatostatin analog octreotide. J Endocrinol 216: 87-97.
- Lepiarczyk E, Bossowska A, Kaleczyc J, Majewska M, Gonkowski S, et al. (2017) The Influence of Tetrodotoxin (TTX) on the Distribution and Chemical Coding of Caudal Mesenteric Ganglion (CaMG) Neurons Supplying the Porcine Urinary Bladder. Mar Drugs 15: E101.
- Li X, Cai L, Xu H, Geng C, Lu J, et al. (2016) Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am J Physiol Gastrointest Liver Physiol 311: G954-G963.
- Liu R, Wei N, Guo W, Qiang Q, Li X, et al. (2013) Octreotide alleviates obesity by reducing intestinal glucose absorption and inhibiting low-grade inflammation Eur J Nutr 52: 1067-1075.
- Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG (2001) The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl 234: 29-40.
- Van Bergeijk JD, Wilson JH (1997) Somatostatin in inflammatory bowel disease. Mediators Inflamm 6: 303-309.
- Low MJ (2004) The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract Res Clin Endocrinol Metab 18: 607-622.
- Palus K, Bulc M, Calka J (2017) Changes in Somatostatin-Like Immunoreactivity in the Sympathetic Neurons Projecting to the Prepyloric Area of the Porcine Stomach Induced by Selected Pathological Conditions. Biomed Res Int 2017: 9037476.
- Pidsudko Z, Kaleczyc J, Wasowicz K, Sienkiewicz W, Majewski M, et al. (2008) Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J Comp Pathol 138: 23-31.
- Pisarek H, Pawlikowski M, Kunert-Radek J, Radek M (2009) Expression of somatostatin receptor subtypes in human pituitary adenomas - immunohistochemical studies. Endokrynol Pol 60: 240-251.
- Pompolo S, Furness JB (1998) Quantitative analysis of inputs to somatostatin immunoreactive descending interneurons in the myenteric plexus of the guinea-pig small intestine. Cell Tissue Res 294: 219-226.
- Rychlik A, Gonkowski S, Nowicki M, Calka J (2015) Cocaine- and amphetamine-regulated transcript immunoreactive nerve fibres in the mucosal layer of the canine gastrointestinal tract under physiological conditions and in inflammatory bowel disease. Vet Med 60:361-367.
- Rychlik A, Gonkowski S, Nowicki M, Calka J (2017) Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can J Vet Res 81: 129-136.
- Rychlik A, Gonkowski S, Nowicki M, Calka J, Szweda M (2015) Galanin - immunoreactive nerve fibers in the mucosal layer of the canine gastrointestinal tract during inflammatory bowel disease. B Vet I Pulawy 59: 143-148.
- Scalera G, Tarozzi G (1998) Somatostatin administration modifies food intake, body weight and gut motility in rat. Peptides 19: 991-997.
- Shaib W, Mitchell K, Saif MW (2010) Amelioration of Symptoms and Reduction of VIP Levels after Hepatic Artery Chemoembolization in a Patient with Sandostatin Resistant VIPoma. Yale J Biol Med 83: 27-33.
- Thoden J, Potthoff A, Bogner JR, Brockmeyer NH, Esser S, et al. (2013) Therapy and prophylaxis of opportunistic infections in HIV-infected patients: a guideline by the German and Austrian AIDS societies (DAIG/ÖAG) (AWMF 055/066). Infection 41:91-115.
- Locher C, Tipold A, Welle M, Busato A, Zurbriggen A, et al. (2001) Quantitative assessment of mast cells and expression of IgE protein and mRNA for IgE and interleukin 4 in the gastrointestinal tract of healthy dogs and dogs with inflammatory bowel disease. Am J Vet Res 62: 211-216.
- Van Op den Bosch J, Adriaensen D, Van Nassauw L, Timmermans JP (2009) The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regul Pept 156: 1-8.
- Van Op den Bosch J, Lantermann K, Torfs P, Van Marck E, Van Nassauw L, et al. (2008) Distribution and expression levels of somatostatin and somatostatin receptors in the ileum of normal and acutely Schistosoma mansoni-infected SSTR 2 knockout/lacZ knockin mice. Neurogastroenterol Motil 20: 566-575.
- Wang J, Cao DY, Guo Y, Ma SJ, Luo R, et al. (2011) Octreotide inhibits capsaicin-induced activation of C and Aδ afferent fibres in rat hairy skin in vivo. Clin Exp Pharmacol Physiol 38: 521-527.
- Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, et al. (2010) Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 24: 10-26.
- Wojtkiewicz J, Rowniak M, Crayton R, Majewski M, Gonkowski S (2012) Chemical coding of zinc-enriched neurons in the intramural ganglia of the porcine jejunum. Cell Tissue Res 350: 215-223.
- Wojtkiewicz J, Rowniak M, Gonkowski S, Crayton R, Majewski M, et al. (2012) Proliferative Enteropathy (PE)—Induced Changes in the Calbindin-Immunoreactive (CB-IR) Neurons of Inferior Mesenteric Ganglion Supplying the Descending Colon in the Pig. J Mol Neurosci 48: 757-765.
- Yamamoto H, Morise K, Kusugami K, Furusawa A, Konagaya T, et al. (1996) Abnormal neuropeptide concentration in rectal mucosa of patients with inflammatory bowel disease. J Gastroenterol 31: 525-532.
- Zalecki M (2012) Localization and neurochemical characteristics of the extrinsic sympathetic neurons projecting to the pylorus in the domestic pig. J Chem Neuroanat 43: 1-13.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences