A Rare Case of Giant Amyloid Goiter: A Case Report and Review of Literature
Carl Christofer Juhlin, Fredrik Karlsson and Robert Bränström
Carl Christofer Juhlin1,2 and Fredrik Karlsson3,4*
1Department of Oncology and Pathology, Karolinska Institutet, Stockholm, Sweden
2Department of Cytology and Pathology, Karolinska University Hospital, Stockholm, Sweden
3Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden
4Department of Breast, Endocrine Tumors and Sarcoma, Karolinska University Hospital, Stockholm, Sweden
- *Corresponding Author:
- Fredrik Karlsson
Endocrine and Sarcoma Surgery Unit
Karolinska University Hospital
D2:02, 17176, Stockholm, Sweden
Tel: +46-8-517 79213
E-mail: robert.branstrom@ki.se
Received Date: April 22, 2019; Accepted Date: May 17, 2019; Published Date: May 24, 2019
Citation: Juhlin CC, Karlsson F (2019) A Rare Case of Giant Amyloid Goiter: A Case Report and Review of Literature. Med Case Rep Vol.5 No.2:100.
Abstract
Background: Amyloid goiter (AG) is an exceedingly rare cause of an enlarged thyroid gland associated to either primary or secondary forms of amyloidosis. We present the development of AG in a euthyroid patient with secondary Amyloid A (AA) amyloidosis, chronic kidney failure and a previous history of a spinal cord sarcoma.
Patient findings: The patient was a 59 year-old male who presented with a slowly progressive enlargement of both thyroid lobes, eventually causing significant tracheal displacement, dysphagia and dyspnea. The previous patient history, the spectacular size in addition to the lipomatous appearance on a cervical CT scan raised some concern that the lesion was in fact a slow-growing liposarcoma of the thyroid. A fine-needle aspiration biopsy identified scarce thyrocytes intermingled with adipose tissue fragments and multiple amyloid deposits. Following a total thyroidectomy, the pathology report was consistent with AG, a diagnosis supported by the occurrence of a remarkable adipose tissue metaplasia adjoined by atrophic follicles and findings of amorphous substances positive for Congo Red staining and Amyloid A (AA) protein immunoreactivity. Liposarcoma and medullary thyroid carcinoma was ruled out. The patient was discharged from further surgical follow-up, and is currently well.
Discussion and conclusion: AG should always be suspected in systemic amyloidosis patients with a pronounced enlargement of the thyroid gland. The entity can be suspected already by radiology, but the cytological and histological identification of amyloid deposits are key attributes for establishing the correct diagnosis.
Keywords
Amyloid; Goiter; Surgery; Pathology
Introduction
Amyloid depositions in the thyroid gland are most commonly calcitonin-derived and observed in conjunction with medullary thyroid carcinoma (MTC), and focal amyloid depositions might also be seen in conventional multinodular goiters. Even so, this deposit seldom causes a significant contribution to the thyroid enlargement. A clinically significant enlargement of the thyroid owing to amyloid depositions unrelated to calcitonin is an extremely rare condition defined by Beckman in the mid-1800s and by Eiselberg in 1904 [1]. These authors conceived the term “amyloid goiter” (AG), a disorder that has been coupled to either a primary or secondary forms of amyloidosis [2]. Primary amyloidosis is caused by systemic amyloid disease unrelated to various chronic conditions, whereas secondary amyloidosis is caused by amyloid aggregates derived from predisposing conditions (chronic inflammatory diseases, infections and hematological disorders) [3-9].
Clinically detectable goiter due to amyloid deposits is extremely rare, and most cases are not diagnosed prior to surgery. At present, less than 100 AG cases have been presented in literature, and most cases are derived from primary amyloidosis [6,9-15]. In this report, we describe a patient with secondary amyloidosis and AG. We discuss the differential diagnoses, possible pitfalls and review the current literature.
Case Report
The patient is a 59-year old non-smoking Caucasian male without any family history of thyroid disorders. He presented with complaints of a gradually increasing swelling in the anterior neck and was first seen at the local hospital, but was subsequently referred to our department due to the alarming size of the goiter. In addition, the patient displayed a change in voice over the past 10-12 months, combined with a history of supine dyspnea as well as dysphagia. During the examination, a lean but otherwise normally built male with a length of 160 cm and weight of 47 kg is seen. The body-mass index (BMI) was 18.4 kg/m2.
A thorough review of the patient ’ s medical history was undertaken. In the early months of 1986, the patient developed a sarcoma of the spinal cord that was surgically resected followed by combined chemo- and radiotherapy. Some 10 years after oncological treatment, the patient developed a slowly deteriorating chronic kidney failure. A kidney biopsy revealed renal amyloidosis with presence of AA protein visualized through immunohistochemistry. The patient was treated with methotrexate and hydrocortisone in intervals, but even so the kidney failure progressed, and between 1998-2001 the patient was enrolled in hemodialysis. In 2001, the patient received a kidney transplant from a living donor and was put on tacrolimus and prednisolone. The kidney graft is still functional.
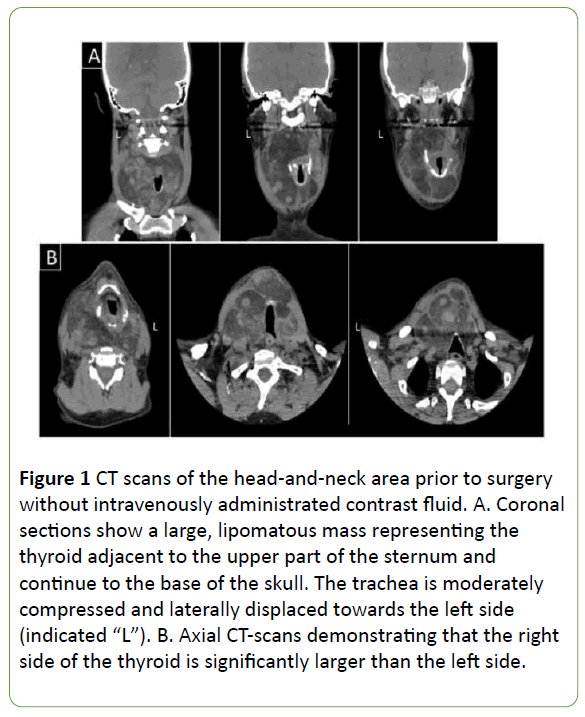
Simultaneously, the patient developed a goiter that slowly progressed. At the start, symptoms were mild, but slowly progressed. In 2016, the patient was referred from his nearby county hospital to the Karolinska University Hospital due to a large neck mass. On physical examination, a 15 × 10 cm large mass involving both the thyroid lobes was palpable in the neck, with a slight overweight to the right side. Examination with blood samples (Table 1), ultrasound and CT scans (Figure 1) showed a sizeable non-toxic goiter measuring at least 14 × 8 × 8 cm (height vs. width vs. depth) on the right side and 9 × 6 × 6 cm on the left side.
| Parameters | Prior to Surgery | 2-h post-operative | 4 weeks follow-up | Units | Reference limits | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| B-Hemoglobulin | 130 | - | - | g/L | 134 | 170 |
| B-Erytocytes | 4.1 | - | - | 1 × 1012/L | 4.2 | 5.7 |
| Erc(B)-MCV | 94 | - | - | fL | 82 | 98 |
| Erc(B)-MCH | 32 | - | - | pg | 27 | 33 |
| B-Leukocytes | 12.4 | - | - | 1 × 109/L | 3.5 | 8.8 |
| B-Trombocytes | 305 | - | - | 1 × 109/L | 145 | 348 |
| P-PK(INR) | 1 | - | - | INR | - | <1.2 |
| P-APT-time | 22 | - | - | s | 20 | 30 |
| P-PTH | 5.2 | 1.3 | 2.8 | pmol/L | 1.6 | 6 |
| P-TSH | 0.11 | - | 0.8 | mE/L | 0.3 | 4.2 |
| P-Thyroxine (free) | 18 | - | 16 | pmol/L | 12 | 22 |
| P-Sodium | 141 | - | - | mmol/L | 137 | 145 |
| P-Potassium | 4.1 | - | - | mmol/L | 3.5 | 4.6 |
| P-Calcium (free) | 1.34 | - | 1.28 | mmol/L | 1.15 | 1.33 |
| P-Creatinine | 129 | - | - | mmol/L | - | <100 |
| Pt-eGFR | 52 | - | - | mL/min/1.7 | >60 | - |
Table 1: Blood samples were taken before thyroidectomy, 2-h post-operative, and at 4 weeks follow-up. Indicated in red are blood samples that deviate from the normal range.
Figure 1: CT scans of the head-and-neck area prior to surgery without intravenously administrated contrast fluid. A. Coronal sections show a large, lipomatous mass representing the thyroid adjacent to the upper part of the sternum and continue to the base of the skull. The trachea is moderately compressed and laterally displaced towards the left side (indicated “L”). B. Axial CT-scans demonstrating that the right side of the thyroid is significantly larger than the left side.
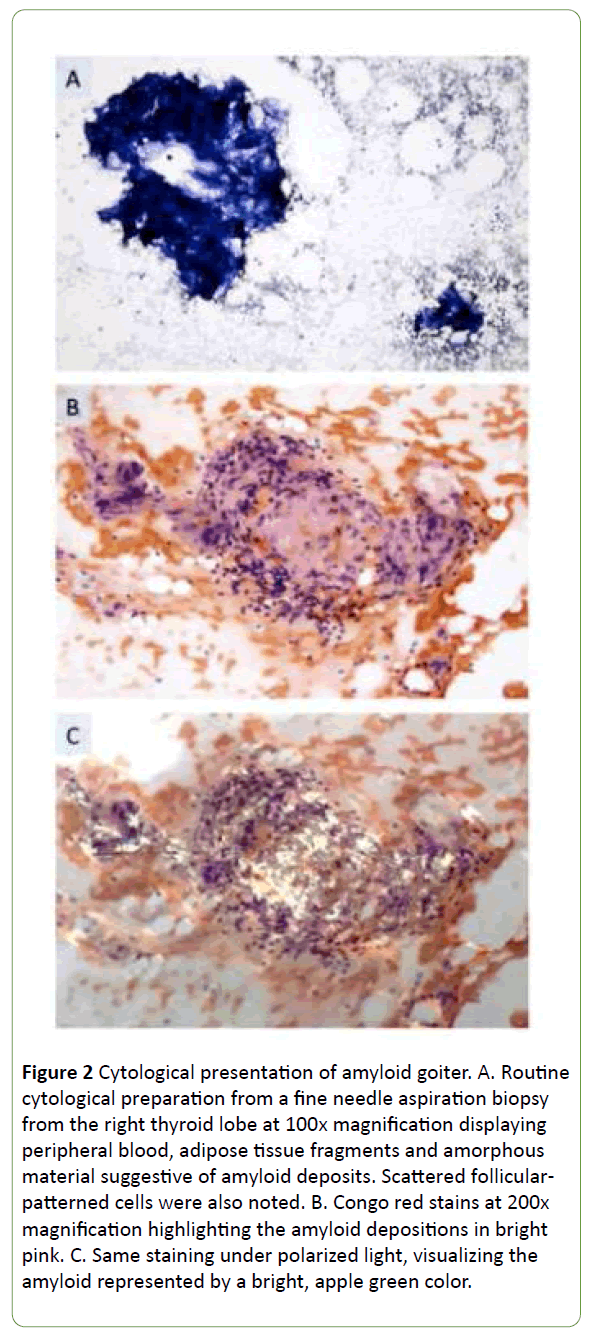
A bilateral fine needle aspiration biopsy (FNAB) was undertaken, and the cytological report indicated the presence of adipose tissue fragments, magenta colored, amorphous deposits and only occasional thyrocytes (Figure 2A). The aggregates stained positive for Congo Red and displayed typical apple green birefringence under polarized light, indicative of amyloid (Figure 2B and 2C).
Figure 2: Cytological presentation of amyloid goiter. A. Routine cytological preparation from a fine needle aspiration biopsy from the right thyroid lobe at 100x magnification displaying peripheral blood, adipose tissue fragments and amorphous material suggestive of amyloid deposits. Scattered follicular-patterned cells were also noted. B. Congo red stains at 200x magnification highlighting the amyloid depositions in bright pink. C. Same staining under polarized light, visualizing the amyloid represented by a bright, apple green color.
A total thyroidectomy was performed without pre- or postoperative complications. As a routine, PTH was measured 2-h postoperative to predict and estimate the risk for hypocalcemia (Table 1). PTH was measured to 1.3 pmol/L, which is slightly below reference (normal range 1.6-6 pmol/L) but the patient did not suffer from hypocalcemia, nor did the patient have any symptoms or signs of hypocalcemia.
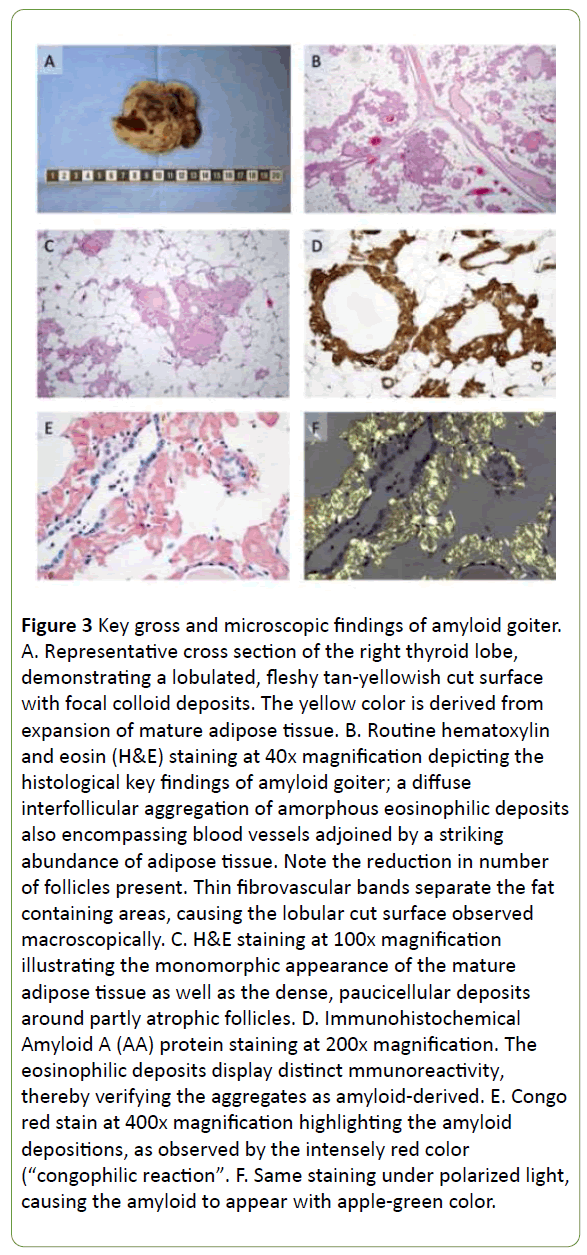
The gross specimen displayed a weight of 322 grams, with the right thyroid lobe measuring 14 × 7 × 7 cm, and a somewhat smaller left lobe measuring 9 × 6 × 5 cm. The cut surface of both lobes was lobulated and yellowish, reminiscent of adipose tissue with degenerative foci as well as areas with macroscopically visible colloid (Figure 3A).
Figure 3: Key gross and microscopic findings of amyloid goiter. A. Representative cross section of the right thyroid lobe, demonstrating a lobulated, fleshy tan-yellowish cut surface with focal colloid deposits. The yellow color is derived from expansion of mature adipose tissue. B. Routine hematoxylin and eosin (H&E) staining at 40x magnification depicting the histological key findings of amyloid goiter; a diffuse interfollicular aggregation of amorphous eosinophilic deposits also encompassing blood vessels adjoined by a striking abundance of adipose tissue. Note the reduction in number of follicles present. Thin fibrovascular bands separate the fat containing areas, causing the lobular cut surface observed macroscopically. C. H&E staining at 100x magnification illustrating the monomorphic appearance of the mature adipose tissue as well as the dense, paucicellular deposits around partly atrophic follicles. D. Immunohistochemical Amyloid A (AA) protein staining at 200x magnification. The eosinophilic deposits display distinct mmunoreactivity, thereby verifying the aggregates as amyloid-derived. E. Congo red stain at 400x magnification highlighting the amyloid depositions, as observed by the intensely red color (“congophilic reaction”. F. Same staining under polarized light, causing the amyloid to appear with apple-green color.
Upon microscopic examination, a striking decrease of thyrocytes was seen apart from the occasional manifestation of single, dilated follicular structures. The parenchyma was crowded with mature adipose tissue, and the remaining thyrocytes as well as blood vessels were encompassed by a eosinophilic and amorphous material reminiscent of amyloid (Figures 3B and 3C). Upon immunohistochemical evaluation, these amyloid deposits were positive for AA (Figure 3D), and an auxiliary Congo red stain was similarly positive (Figures 3E and 3F). There was no immunoreactivity noted for calcitonin, thereby excluding MTC. The final diagnosis was consistent with AG.
Discussion
Amyloidosis is a multifaceted condition with a heterogeneous etiological background, although one common denominator is found; the extracellular accumulation of aberrantly folded protein components causing amyloid fibrils. The thyroid can be asymptomatically involved by amyloid substance in 30-80% of patients with primary or secondary amyloidosis [1,16]. In more than 50% of patients with medullary thyroid carcinoma, amyloid may also be encountered in the thyroid [2,3]. Still, amyloid goiter, which is a symptomatic mass or clinically detectable thyroid enlargement due to amyloid deposition, is considered as a rarity [17]. The diverse symptomatology depends on the specific organ predilection for different amyloidosis subtypes, and the disease therefore carries a broad clinical spectrum regarding patient presentation. Common types of secondary (or “systemic”) amyloidosis include the subtypes amyloid light-chain (AL), amyloid A (AA), familial amyloidosis (AF) and amyloid A hereditary (AH) amyloidosis [18]. Our patient was previously diagnosed with AA amyloidosis via a core needle biopsy from the kidney, and the amyloid deposits in the thyroid specimen exhibited a distinct AA immunoreactivity – supporting the diagnosis of AA amyloidosis. This specific form of the disease is caused by aberrant extracellular accumulations of serum amyloid A (sAA) protein, which in turn instigated by chronic inflammation. Given its role as an acute phase reactant, it is released from the liver upon stimulation by pro-inflammatory cytokines. During long-lasting inflammatory conditions, the levels of sAA will increase, and the protein will bind to other peptides and glycocomponents of the extracellular matrix – which is thought to activate fibrillogenesis, leading to the formation of amyloid deposits [19]. Although AA amyloidosis is a common form of systemic amyloidosis in patients with chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, the reason why AG is so seldom encountered in these patient populations is not known [4,5,7].
Our case is intriguing from many aspects. Firstly, our patient exhibited a previous history of a spinal cord sarcoma, and the exaggerated thyroid enlargement was initially suspected to constitute a metastatic lesion. The preoperative cytology however indicated the presence of amyloid deposits, which was not entirely surprising given the patient’s previous diagnosis of AA amyloidosis. Secondly, since the patient also exhibited chronic renal failure, one could in theory suspect dialysis-related amyloidosis as a contributor of the AG development [20]. In this disease, the amyloidogenic protein is β2-microglobulin, and theses amyloid aggregates are known to cause destructive arthopathies and carpal tunnel syndrome – symptoms that our patient lacked altogether. As we were able to identify the AG deposits as AA-derived by immunohistochemistry, there is little suspicion that the patient also suffered from dialysis-associated amyloidosis. Another fascinating feature of this case is the overall kinetics. In most publications, the authors describe AG presenting as a rapidly occurring enlargement of the thyroid gland, which was not the case for our patient – as the goiter was present already some 10-15 years before surgery [21,22]. This suggests that the process governing the deposition of amyloid was gradual, causing the associated adipose tissue metaplasia to develop slowly, which has been observed in occasional cases previously [9].
Conclusion
Since the symptomatology and disease severity varies with the different underlying causes of AA amyloidosis, it is possible that a persistent and discreet inflammation could lead to a less pronounced AG than various high-grade inflammatory conditions. In our patient, the actual cause of the systemic amyloidosis is not known. We conclude that AG should be suspected in amyloidosis patients with a prominent enlargement of the thyroid gland, even if the growth rate is diminutive. Moreover, AA immunohistochemistry was helpful in determining the systemic nature of the amyloidosis.
References
- Arean VM, Klein RE (1961). Amyloid goiter: A eeview of the literature and report of a case. Am J Clin Pathol 36: 341-355.
- Nosé V (2018) Diagnostic pathology. Endocrine. (2nd edn). Philadelphia, PA: Elsevier.
- Altiparmak MR, Pamuk ON, Pamuk GE, Apaydin S, Ataman R, et al. (2002) Amyloid goitre in familial Mediterranean fever: report on three patients and review of the literature. Clin Rheumatol 21: 497-500.
- Uzum G, Kaya FO, Uzum AK, Kucukyilmaz M, Gunes ME, et al. (2013) Amyloid goiter associated with amyloidosis secondary to rheumatoid arthritis. Case Rep Med 2013: 792413.
- Ozemir IA, Bilgic C, Bayraktar B, Aslan S, Zemheri E, et al. (2014) Amyloid goiter related with Crohn’s disease: A rare association: Amyloid goiter secondary to Crohn’s disease. Int J Surg Case Rep 5: 480-483.
- Kazdaghli Lagha E, M’sakni I, Bougrine F, Laabidi B, Ben Ghachem D, et al. (2010) Amyloid goiter: first manifestation of systemic amyloidosis. Eur Ann Otorhinolaryngol Head Neck Dis 127: 108-110.
- Aydin B, Koca YS, Koca T, Yildiz I, Gerek Celikden S, et al. (2016) Amyloid Goiter Secondary to Ulcerative Colitis. Case Rep Endocrinol 2016: 3240585.
- D’Antonio A, Franco R, Sparano L, Terzi G, Pettinato G (2000) Amyloid goiter: The first evidence in secondary amyloidosis. Report of five cases and review of literature. Adv Clin Path 4: 99-106.
- Law JH, Dean DS, Scheithauer B, Earnest F, Sebo TJ, et al. (2013) Symptomatic amyloid goiters: report of five cases. Thyroid 23: 1490-1495.
- Amado JA, Ondiviela R, Palacios S, Casanova D, Manzanos J, et al. (1982) Fast growing goitre as the first clinical manifestation of systemic amyloidosis. Postgrad Med J 58: 171-172.
- Pérez Fontán FJ, Mosquera Osés J, Pombo Felipe F, Rodriguez Sanchez I, Arnaiz Peña S (1992) Amyloid goiter in a child--US, CT and MR evaluation. Pediatr Radiol 22: 393-394.
- Cabrejas Gómez MDC, González Cabrera N, Gómez González C, Bergara Elorza S (20145) Amyloid goiter as an initial manifestation of systemic amyloidosis. Reumatol Clin 11: 404-405.
- Siddiqui MA, Gertz M, Dean D (2007) Amyloid goiter as a manifestation of primary systemic amyloidosis. Thyroid 17: 77-80.
- Ozdemir D, Dagdelen S, Erbas T (2010) Endocrine involvement in systemic amyloidosis. Endocr Pract 16: 1056-1063.
- Wang AR, Baloch ZW, Montone KT (2016) Amyloid Goiter in a Patient with Progressive Thyromegaly. Endocr Pathol 27: 76-78.
- Briggs GW (1961) Amyloidosis. Ann Intern Med 55: 943-957.
- Villamil CF, Massimi G, D’Avella J, Cole SR (2000) Amyloid goiter with parathyroid involvement: a case report and review of the literature. Arch Pathol Lab Med 124: 281-283.
- Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet 387: 2641-2654.
- Papa R, Lachmann HJ (2018) Secondary Amyloidosis. Rheum Dis Clin North Am 44: 585-603.
- Ohashi K (2001) Pathogenesis of beta2-microglobulin amyloidosis. Pathol Int 51: 1-10.
- Joung KH, Park JY, Kim KS, Koo BS (2014) Primary amyloid goiter mimicking rapid growing thyroid malignancy. Eur Arch Otorhinolaryngol 271: 417-420.
- Ibrahimov M, Yilmaz M, Kilic E, Akil F, Rasidov R, et al. (2012) Rapidly progressive thyroid mass: Amyloid goiter. J Craniofac Surg 23: e555-e556.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences